Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate
- PMID: 20418863
- PMCID: PMC2919753
- DOI: 10.1038/nmat2732
Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate
Abstract
Stem cells sense and respond to the mechanical properties of the extracellular matrix. However, both the extent to which extracellular-matrix mechanics affect stem-cell fate in three-dimensional microenvironments and the underlying biophysical mechanisms are unclear. We demonstrate that the commitment of mesenchymal stem-cell populations changes in response to the rigidity of three-dimensional microenvironments, with osteogenesis occurring predominantly at 11-30 kPa. In contrast to previous two-dimensional work, however, cell fate was not correlated with morphology. Instead, matrix stiffness regulated integrin binding as well as reorganization of adhesion ligands on the nanoscale, both of which were traction dependent and correlated with osteogenic commitment of mesenchymal stem-cell populations. These findings suggest that cells interpret changes in the physical properties of adhesion substrates as changes in adhesion-ligand presentation, and that cells themselves can be harnessed as tools to mechanically process materials into structures that feed back to manipulate their fate.
Figures


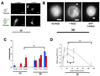
 ) and αV (
) and αV ( ) integrin binding to RGD-biotin presented at varying density by either 2D or 3D alginate matrices (* p < 0.01, t-test). (D). α5-integrin-RGD bond formation in matrices with varying stiffness presenting either 37 µM (
) integrin binding to RGD-biotin presented at varying density by either 2D or 3D alginate matrices (* p < 0.01, t-test). (D). α5-integrin-RGD bond formation in matrices with varying stiffness presenting either 37 µM ( ) or 754 µM (
) or 754 µM ( ) RGD-biotin (* p < 0.01, t-test). α5-integrin binding to matrices presenting 754µM RGE-biotin was negligible. Error bars are SEM (n = 4–5). Scale bars: (A), 20µm; (B), 5µm.
) RGD-biotin (* p < 0.01, t-test). α5-integrin binding to matrices presenting 754µM RGE-biotin was negligible. Error bars are SEM (n = 4–5). Scale bars: (A), 20µm; (B), 5µm.
 ), or cells treated with either 20mM BDM (
), or cells treated with either 20mM BDM ( ) or 20µg/mL cyclohexamide (▲) encapsulated into matrices presenting 37µM RGD. FRET analyses of cell-RGD bonds were performed 2 hr after encapsulating cells, and E values shown are for hydrogels at the time of cell encapsulation. Scale bars: (B); 10µm.
) or 20µg/mL cyclohexamide (▲) encapsulated into matrices presenting 37µM RGD. FRET analyses of cell-RGD bonds were performed 2 hr after encapsulating cells, and E values shown are for hydrogels at the time of cell encapsulation. Scale bars: (B); 10µm.
 ) or 150 µM (
) or 150 µM ( ) RGD were used to calculate h. (C). Schematic depicting enhanced cell-RGD bond formation due to nanoscale RGD clustering mediated by cell traction forces. (D). Schematic of FRET assay to monitor cell-traction mediated nanoscale RGD-clustering of RGD-CFsC and RGD-TAMRA attached to different alginate chains. (E). FRET measurements of nanoscale RGD-clustering by encapsulated mMSC (* p < 0.01 compared to other conditions, Holm-Bonferonni test). FRET analyses of integrin ligation and nanoscale-RGD reorganization were performed 2 hr after encapsulating cells, and E values shown are for hydrogels at the time of cell encapsulation. Schematic drawings are not meant to be to scale. Error bars are SEM for calculated protrusion depth calculations (n = 3–5) and SD (n = 3) for clustering measurements.
) RGD were used to calculate h. (C). Schematic depicting enhanced cell-RGD bond formation due to nanoscale RGD clustering mediated by cell traction forces. (D). Schematic of FRET assay to monitor cell-traction mediated nanoscale RGD-clustering of RGD-CFsC and RGD-TAMRA attached to different alginate chains. (E). FRET measurements of nanoscale RGD-clustering by encapsulated mMSC (* p < 0.01 compared to other conditions, Holm-Bonferonni test). FRET analyses of integrin ligation and nanoscale-RGD reorganization were performed 2 hr after encapsulating cells, and E values shown are for hydrogels at the time of cell encapsulation. Schematic drawings are not meant to be to scale. Error bars are SEM for calculated protrusion depth calculations (n = 3–5) and SD (n = 3) for clustering measurements.
Comment in
-
The life of a cell: probing the complex relationships with the world.Cell Stem Cell. 2010 Jun 4;6(6):499-501. doi: 10.1016/j.stem.2010.05.009. Cell Stem Cell. 2010. PMID: 20569684 No abstract available.
References
-
- Passier R, van Laake LW, Mummery CL. Stem-cell based therapy and lessons from the heart. Nature. 2008;453:322–329. - PubMed
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Geiger B, Bershadsky A. Exploring the neighborhood: adhesion-coupled mechanosensors. Cell. 2002;110:139–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

