PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ-TIF2
- PMID: 20418886
- PMCID: PMC3039870
- DOI: 10.1038/nm.2122
PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ-TIF2
Abstract
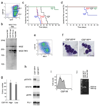
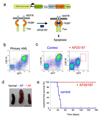
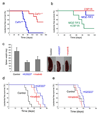
Leukemias and other cancers possess self-renewing stem cells that help to maintain the cancer. Cancer stem cell eradication is thought to be crucial for successful anticancer therapy. Using an acute myeloid leukemia (AML) model induced by the leukemia-associated monocytic leukemia zinc finger (MOZ)-TIF2 fusion protein, we show here that AML can be cured by the ablation of leukemia stem cells. The MOZ fusion proteins MOZ-TIF2 and MOZ-CBP interacted with the transcription factor PU.1 to stimulate the expression of macrophage colony-stimulating factor receptor (CSF1R, also known as M-CSFR, c-FMS or CD115). Studies using PU.1-deficient mice showed that PU.1 is essential for the ability of MOZ-TIF2 to establish and maintain AML stem cells. Cells expressing high amounts of CSF1R (CSF1R(high) cells), but not those expressing low amounts of CSF1R (CSF1R(low) cells), showed potent leukemia-initiating activity. Using transgenic mice expressing a drug-inducible suicide gene controlled by the CSF1R promoter, we cured AML by ablation of CSF1R(high) cells. Moreover, induction of AML was suppressed in CSF1R-deficient mice and CSF1R inhibitors slowed the progression of MOZ-TIF2-induced leukemia. Thus, in this subtype of AML, leukemia stem cells are contained within the CSF1R(high) cell population, and we suggest that targeting of PU.1-mediated upregulation of CSF1R expression might be a useful therapeutic approach.
Figures

References
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Borrow J, et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

