Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice
- PMID: 20418887
- PMCID: PMC2907076
- DOI: 10.1038/nm.2125
Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice
Abstract
Obsessive-compulsive disorder (OCD) is a common psychiatric disorder defined by the presence of obsessive thoughts and repetitive compulsive actions, and it often encompasses anxiety and depressive symptoms. Recently, the corticostriatal circuitry has been implicated in the pathogenesis of OCD. However, the etiology, pathophysiology and molecular basis of OCD remain unknown. Several studies indicate that the pathogenesis of OCD has a genetic component. Here we demonstrate that loss of a neuron-specific transmembrane protein, SLIT and NTRK-like protein-5 (Slitrk5), leads to OCD-like behaviors in mice, which manifests as excessive self-grooming and increased anxiety-like behaviors, and is alleviated by the selective serotonin reuptake inhibitor fluoxetine. Slitrk5(-/-) mice show selective overactivation of the orbitofrontal cortex, abnormalities in striatal anatomy and cell morphology and alterations in glutamate receptor composition, which contribute to deficient corticostriatal neurotransmission. Thus, our studies identify Slitrk5 as an essential molecule at corticostriatal synapses and provide a new mouse model of OCD-like behaviors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

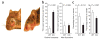

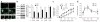
Comment in
-
SLITRK5, a protein that links striatal deficits to OCD-like behaviours in mice.Clin Genet. 2010 Oct;78(4):350-2. doi: 10.1111/j.1399-0004.2010.01507.x. Clin Genet. 2010. PMID: 20718795 No abstract available.
References
-
- Miguel EC, et al. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol Psychiatry. 2005;10:258–275. - PubMed
-
- Karno M, Golding JM, Sorenson SB, Burnam MA. The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry. 1988;45:1094–1099. - PubMed
-
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. - PubMed
-
- Clifford CA, Murray RM, Fulker DW. Genetic and environmental influences on obsessional traits and symptoms. Psychol Med. 1984;14:791–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 MH088814/MH/NIMH NIH HHS/United States
- MH079513/MH/NIMH NIH HHS/United States
- U01 HL066952/HL/NHLBI NIH HHS/United States
- P50 MH079513/MH/NIMH NIH HHS/United States
- NS052819/NS/NINDS NIH HHS/United States
- HL66592/HL/NHLBI NIH HHS/United States
- RC1 AI080309/AI/NIAID NIH HHS/United States
- R01 NS052819/NS/NINDS NIH HHS/United States
- R01 HL097797/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- AI080309/AI/NIAID NIH HHS/United States
- HL097797/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

