TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB
- PMID: 20418905
- PMCID: PMC2892027
- DOI: 10.1038/onc.2010.116
TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB
Abstract
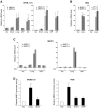
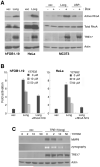
Aneurysmal bone cyst (ABC) is an aggressive, pediatric bone tumor characterized by extensive destruction of the surrounding bone. Although first described over 60 years ago, its molecular etiology remains poorly understood. Recent work revealed that ABCs harbor translocation of TRE17/USP6, leading to its transcriptional upregulation. TRE17 encodes a ubiquitin-specific protease (USP), and a TBC domain that mediates binding to the Arf6 GTPase. However, the mechanisms by which TRE17 overexpression contributes to tumor pathogenesis, and the role of its USP and TBC domains, are unknown. ABCs are characterized by osteolysis, inflammatory recruitment and extensive vascularization, the processes in which matrix proteases have a prominent role. This led us to explore whether TRE17 regulates the production of matrix metalloproteinases (MMPs). In this study we show that TRE17 is sufficient to induce expression of MMP-9 and MMP-10, in a manner requiring its USP activity, but not its ability to bind Arf6. TRE17 induces transcription of MMP-9 through activation of nuclear factor-kappaB (NF-kappaB), mediated in part by the GTPase RhoA and its effector kinase, ROCK. Furthermore, xenograft studies show that TRE17 induces formation of tumors that reproduce multiple features of ABC, including a high degree of vascularization, with an essential role for the USP domain. In sum, these studies reveal that TRE17 is sufficient to initiate tumorigenesis, identify MMPs as novel TRE17 effectors that likely contribute to ABC pathogenesis and define the underlying signaling mechanism of their induction.
Figures




References
-
- Althof PA, Ohmori K, Zhou M, Bailey JM, Bridge RS, Nelson M, et al. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: aberrations of 17p mapped to 17p13.2 by fluorescence in situ hybridization. Mod Pathol. 2004;17:518–525. - PubMed
-
- Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695:189–207. - PubMed
-
- Anwar KN, Fazal F, Malik AB, Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol. 2004;173:6965–6972. - PubMed
-
- Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, et al. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum. 2006;54:3244–3253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

