Abuse Potential of Soma: the GABA(A) Receptor as a Target
- PMID: 20419052
- PMCID: PMC2858432
Abuse Potential of Soma: the GABA(A) Receptor as a Target
Abstract
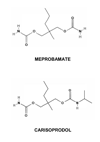
Soma(®) (carisoprodol) is an increasingly abused, centrally-acting muscle relaxant. Despite the prevalence of carisoprodol abuse, its mechanism of action remains unclear. Its sedative effects, which contribute to its therapeutic and recreational use, are generally attributed to the actions of its primary metabolite, meprobamate, at GABA(A) receptors (GABA(A)R). Meprobamate is a controlled substance at the federal level; ironically, carisoprodol is not currently classified as such. Using behavioral and molecular pharmacological approaches, we recently demonstrated carisoprodol, itself, is capable of modulating GABA(A)R function in a manner similar to central nervous system depressants. Its functional similarities with this highly addictive class of drugs may contribute to the abuse potential of carisoprodol. The site of action of carisoprodol has not been identified; based on our studies, interaction with benzodiazepine or barbiturate sites is unlikely. These recent findings, when coupled with numerous reports in the literature, support the contention that the non-controlled status of carisoprodol should be reevaluated.
Conflict of interest statement
No potential conflicts of interest to disclose.
Figures
References
-
- Luo X, Pietrobon R, Curtis LH, et al. Prescription of nonsteroidal anti-inflammatory drugs and muscle relaxants for back pain in the United States. Spine. 2004;29:E531–E537. - PubMed
-
- United States Department of Justice Drug Enforcement Administration Office of Diversion Control. Drugs and chemicals of concern--carisoprodol [internet] 2009. Jun [cited 2009 Aug 12]. Available from: http://www.deadiversion.usdoj.gov/drugs_concern/carisoprodol.htm.
-
- Sikdar S, Basu D, Malhotra AK, et al. Carisoprodol abuse: a report from India. Acta Psychiatr Scand. 1993;88:302–303. - PubMed
-
- Rust GS, Hatch R, Gums JG. Carisoprodol as a drug of abuse. Arch Fam Med. 1993;2:429–432. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
