The AGBNP2 Implicit Solvation Model
- PMID: 20419084
- PMCID: PMC2857935
- DOI: 10.1021/ct900234u
The AGBNP2 Implicit Solvation Model
Abstract
The AGBNP2 implicit solvent model, an evolution of the Analytical Generalized Born plus Non-Polar (AGBNP) model we have previously reported, is presented with the aim of modeling hydration effects beyond those described by conventional continuum dielectric representations. A new empirical hydration free energy component based on a procedure to locate and score hydration sites on the solute surface is introduced to model first solvation shell effects, such as hydrogen bonding, which are poorly described by continuum dielectric models. This new component is added to the Generalized Born and non-polar AGBNP terms. Also newly introduced is an analytical Solvent Excluded Volume (SEV) model which improves the solute volume description by reducing the effect of spurious high-dielectric interstitial spaces present in conventional van der Waals representations. The AGBNP2 model is parametrized and tested with respect to experimental hydration free energies of small molecules and the results of explicit solvent simulations. Modeling the granularity of water is one of the main design principles employed for the the first shell solvation function and the SEV model, by requiring that water locations have a minimum available volume based on the size of a water molecule. It is shown that the new volumetric model produces Born radii and surface areas in good agreement with accurate numerical evaluations of these quantities. The results of molecular dynamics simulations of a series of mini-proteins show that the new model produces conformational ensembles in substantially better agreement with reference explicit solvent ensembles than the original AGBNP model with respect to both structural and energetics measures.
Figures





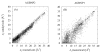

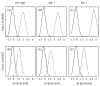


References
-
- Levy RM, Gallicchio E. Annu Rev Phys Chem. 1998;49:531–67. - PubMed
-
- Feig M, Brooks C. Curr Op Struct Biol. 2004;14:217–224. - PubMed
-
- Roux B, Simonson T. Biophysical Chemistry. 1999;78:1–20. - PubMed
-
- Felts AK, Andrec M, Gallicchio E, Levy R. Water and Biomolecules - Physical Chemistry of Life Phenomena. Springer Science; 2008. Protein Folding and Binding: Effective Potentials, Replica Exchange Simulations, and Network Models.
-
- Gallicchio E, Zhang LY, Levy RM. J Comp Chem. 2002;23:517–529. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
