T- and B-cell-mediated protection induced by novel, live attenuated pertussis vaccine in mice. Cross protection against parapertussis
- PMID: 20419113
- PMCID: PMC2855369
- DOI: 10.1371/journal.pone.0010178
T- and B-cell-mediated protection induced by novel, live attenuated pertussis vaccine in mice. Cross protection against parapertussis
Abstract
Background: Despite the extensive use of efficacious vaccines, pertussis still ranks among the major causes of childhood mortality worldwide. Two types of pertussis vaccines are currently available, whole-cell, and the more recent acellular vaccines. Because of reduced reactogenicity and comparable efficacy acellular vaccines progressively replace whole-cell vaccines. However, both types require repeated administrations for optimal efficacy. We have recently developed a live attenuated vaccine candidate, named BPZE1, able to protect infant mice after a single nasal administration.
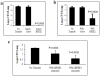
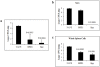
Methodology/principal findings: We determined the protective mechanism of BPZE1-mediated immunity by using passive transfer of T cells and antibodies from BPZE1-immunized mice to SCID mice. Clearance of Bordetella pertussis from the lungs was mediated by both BPZE1-induced antibodies and CD4(+), but not by CD8(+) T cells. The protective CD4(+) T cells comprised IFN-gamma-producing and IL-17-producing subsets, indicating that BPZE1 induces both Th1 and Th17 CD4(+) T cells. In addition, and in contrast to acellular pertussis vaccines, BPZE1 also cross-protected against Bordetella parapertussis infection, but in this case only the transfer of CD4(+) T cells conferred protection. Serum from BPZE1-immunized mice was not able to kill B. parapertussis and did not protect SCID mice against B. parapertussis infection.
Conclusions/significance: The novel live attenuated pertussis vaccine BPZE1 protects in a pre-clinical mouse model against B. pertussis challenge by both BPZE1-induced antibodies and CD4(+) T cell responses. It also protects against B. parapertussis infection. However, in this case protection is only T cell mediated.
Conflict of interest statement
Figures






References
-
- Greenberg DP, von König CH, Heininger U. Health burden of pertussis in infants and hildren. Pediatr Infect Dis J. 2005;24:S39–S43. - PubMed
-
- McIntyre P, Wood N. Pertussis in early infancy: disease burden and preventive strategies. Curr Opin Infect Dis. 2009;22:215–223. - PubMed
-
- Von König CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis. 2002;2:744–750. - PubMed
-
- Crowcroft NS, Stein C, Duclos P, Birmingham M. How best to estimate the global burden of pertussis? Lancet Infect Dis. 2003;3:423–418. - PubMed
-
- Edwards KM. Overview of pertussis: focus on epidemiology, sources of infection, and long term protection after infant vaccination. Pediatr Infect Dis J. 2005;24(Suppl.):S104–S108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

