Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma
- PMID: 20419144
- PMCID: PMC2855320
- DOI: 10.1371/journal.ppat.1000842
Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma
Abstract
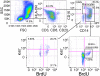
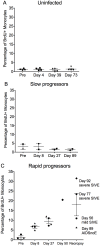
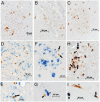
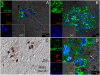
Cells of the myeloid lineage are significant targets for human immunodeficiency virus (HIV) in humans and simian immunodeficiency virus (SIV) in monkeys. Monocytes play critical roles in innate and adaptive immunity during inflammation. We hypothesize that specific subsets of monocytes expand with AIDS and drive central nervous system (CNS) disease. Additionally, there may be expansion of cells from the bone marrow through blood with subsequent macrophage accumulation in tissues driving pathogenesis. To identify monocytes that recently emigrated from bone marrow, we used 5-bromo-2'-deoxyuridine (BrdU) labeling in a longitudinal study of SIV-infected CD8+ T lymphocyte depleted macaques. Monocyte expansion and kinetics in blood was assessed and newly migrated monocyte/macrophages were identified within the CNS. Five animals developed rapid AIDS with differing severity of SIVE. The percentages of BrdU+ monocytes in these animals increased dramatically, early after infection, peaking at necropsy where the percentage of BrdU+ monocytes correlated with the severity of SIVE. Early analysis revealed changes in the percentages of BrdU+ monocytes between slow and rapid progressors as early as 8 days and consistently by 27 days post infection. Soluble CD163 (sCD163) in plasma correlated with the percentage of BrdU+ monocytes in blood, demonstrating a relationship between monocyte activation and expansion with disease. BrdU+ monocytes/macrophages were found within perivascular spaces and SIVE lesions. The majority (80-90%) of the BrdU+ cells were Mac387+ that were not productively infected. There was a minor population of CD68+BrdU+ cells (<10%), very few of which were infected (<1% of total BrdU+ cells). Our results suggest that an increased rate of monocyte recruitment from bone marrow into the blood correlates with rapid progression to AIDS, and the magnitude of BrdU+ monocytes correlates with the severity of SIVE.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Gonzalez-Mejia ME, Doseff AI. Regulation of monocytes and macrophages cell fate. Front Biosci. 2009;14:2413–2431. - PubMed
-
- van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. - PubMed
-
- Tushinski RJ, Oliver IT, Guilbert LJ, Tynan PW, Warner JR, et al. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. - PubMed
-
- Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19MH081835/MH/NIMH NIH HHS/United States
- R01 NS040237/NS/NINDS NIH HHS/United States
- AI040101/AI/NIAID NIH HHS/United States
- P51 RR000164/RR/NCRR NIH HHS/United States
- R01NS37654/NS/NINDS NIH HHS/United States
- RR00164/RR/NCRR NIH HHS/United States
- R01NS40237/NS/NINDS NIH HHS/United States
- R01 NS037654/NS/NINDS NIH HHS/United States
- R01 AI040101/AI/NIAID NIH HHS/United States
- R24 RR016001/RR/NCRR NIH HHS/United States
- U19 MH081835/MH/NIMH NIH HHS/United States
- R01NS06897/NS/NINDS NIH HHS/United States
- RR016001/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

