The direct catalytic asymmetric aldol reaction
- PMID: 20419212
- PMCID: PMC3714873
- DOI: 10.1039/b923537j
The direct catalytic asymmetric aldol reaction
Abstract
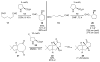
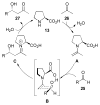
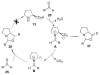
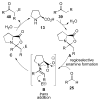
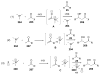
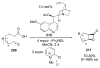
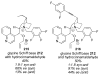
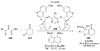
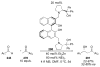
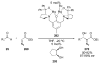
Asymmetric aldol reactions are a powerful method for the construction of carbon-carbon bonds in an enantioselective fashion. Historically this reaction has been performed in a stoichiometric fashion to control the various aspects of chemo-, diastereo-, regio- and enantioselectivity, however, a more atom economical approach would unite high selectivity with the use of only a catalytic amount of a chiral promoter. This critical review documents the development of direct catalytic asymmetric aldol methodologies, including organocatalytic and metal-based strategies. New methods have improved the reactivity, selectivity and substrate scope of the direct aldol reaction and enabled the synthesis of complex molecular targets (357 references).
Figures
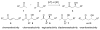

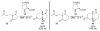
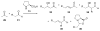



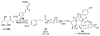
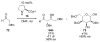




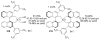
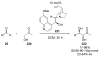


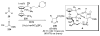
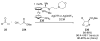



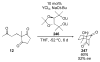









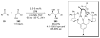

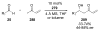
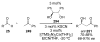
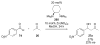




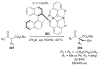


References
-
- Wurtz A. Bull Soc Chim Fr. 1872;17:436.
-
- Mahrwald R, editor. Modern Aldol Reactions. Wiley-VCH; Berlin: 2004.
-
- Trost BM. Science. 1991;254:1471. - PubMed
-
- Fessner W-D. In: Modern Aldol Reactions. Mahrwald R, editor. Wiley-VCH; Berlin: 2004.
-
- Tanaka A, Barbas CF., III . In: Modern Aldol Reactions. Mahrwald R, editor. Wiley-VCH; Berlin: 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

