TonB-dependent transporters: regulation, structure, and function
- PMID: 20420522
- PMCID: PMC3108441
- DOI: 10.1146/annurev.micro.112408.134247
TonB-dependent transporters: regulation, structure, and function
Abstract
TonB-dependent transporters (TBDTs) are bacterial outer membrane proteins that bind and transport ferric chelates, called siderophores, as well as vitamin B(12), nickel complexes, and carbohydrates. The transport process requires energy in the form of proton motive force and a complex of three inner membrane proteins, TonB-ExbB-ExbD, to transduce this energy to the outer membrane. The siderophore substrates range in complexity from simple small molecules such as citrate to large proteins such as serum transferrin and hemoglobin. Because iron uptake is vital for almost all bacteria, expression of TBDTs is regulated in a number of ways that include metal-dependent regulators, σ/anti-σ factor systems, small RNAs, and even a riboswitch. In recent years, many new structures of TBDTs have been solved in various states, resulting in a more complete understanding of siderophore selectivity and binding, signal transduction across the outer membrane, and interaction with the TonB-ExbB-ExbD complex. However, the transport mechanism is still unclear. In this review, we summarize recent progress in understanding regulation, structure, and function in TBDTs and questions remaining to be answered.
Figures
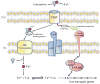

References
-
- Althaus EW, Outten CE, Olson KE, Cao H, O'Halloran TV. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry. 1999;38:6559–69. - PubMed
-
- Angerer A, Braun V. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch Microbiol. 1998;169:483–90. - PubMed
-
- Bagg A, Neilands JB. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987;26:5471–7. - PubMed
-
- Barnard TJ, Wally JL, Buchanan SK. Curr Protoc Protein Sci. Unit 17 9 Chapter 17. 2007. Crystallization of integral membrane proteins. - PubMed
-
- Braun V, Mahren S. Transmembrane transcriptional control (surface signalling) of the Escherichia coli Fec type. FEMS Microbiol Rev. 2005;29:673–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

