Maleic anhydride-modified chicken ovalbumin as an effective and inexpensive anti-HIV microbicide candidate for prevention of HIV sexual transmission
- PMID: 20420669
- PMCID: PMC2888735
- DOI: 10.1186/1742-4690-7-37
Maleic anhydride-modified chicken ovalbumin as an effective and inexpensive anti-HIV microbicide candidate for prevention of HIV sexual transmission
Abstract
Background: Previous studies have shown that 3-hydroxyphthalic anhydride (HP)-modified bovine milk protein, beta-lactoglobulin (beta-LG), is a promising microbicide candidate. However, concerns regarding the potential risk of prion contamination in bovine products and carcinogenic potential of phthalate derivatives were raised. Here we sought to replace bovine protein with an animal protein of non-bovine origin and substitute HP with another anhydride for the development of anti-HIV microbicide for preventing HIV sexual transmission.
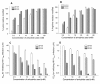
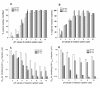
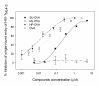
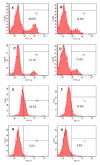
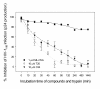
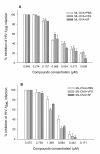
Results: Maleic anhydride (ML), succinic anhydride (SU) and HP at different conditions and variable pH values were used for modification of proteins. All the anhydrate-modified globulin-like proteins showed potent anti-HIV activity, which is correlated with the percentage of modified lysine and arginine residues in the modified protein. We selected maleic anhydride-modified ovalbumin (ML-OVA) for further study because OVA is easier to obtain than beta-LG, and ML is safer than HP. Furthermore, ML-OVA exhibited broad antiviral activities against HIV-1, HIV-2, SHIV and SIV. This modified protein has no or low in vitro cytotoxicity to human T cells and vaginal epithelial cells. It is resistant to trypsin hydrolysis, possibly because the lysine and arginine residues in OVA are modified by ML. Mechanism studies suggest that ML-OVA inhibits HIV-1 entry by targeting gp120 on HIV-1 virions and also the CD4 receptor on the host cells.
Conclusion: ML-OVA is a potent HIV fusion/entry inhibitor with the potential to be developed as an effective, safe and inexpensive anti-HIV microbicide.
Figures



Similar articles
-
Combinations of 3-hydroxyphthalic anhydride-modified ovalbumin with antiretroviral drug-based microbicide candidates display synergistic and complementary effects against HIV-1 infection.J Acquir Immune Defic Syndr. 2011 Apr 15;56(5):384-92. doi: 10.1097/QAI.0b013e31820a4a8d. J Acquir Immune Defic Syndr. 2011. PMID: 21239999 Free PMC article.
-
3-hydroxyphthalic anhydride-modified chicken ovalbumin exhibits potent and broad anti-HIV-1 activity: a potential microbicide for preventing sexual transmission of HIV-1.Antimicrob Agents Chemother. 2010 May;54(5):1700-11. doi: 10.1128/AAC.01046-09. Epub 2010 Mar 1. Antimicrob Agents Chemother. 2010. PMID: 20194691 Free PMC article.
-
3-Hydroxyphthalic anhydride-modified human serum albumin as a microbicide candidate inhibits HIV infection by blocking viral entry.J Antimicrob Chemother. 2013 Mar;68(3):573-6. doi: 10.1093/jac/dks458. Epub 2012 Dec 7. J Antimicrob Chemother. 2013. PMID: 23221626
-
Microbicides for multidrug-resistant and multitropic HIV-1.Curr Opin Investig Drugs. 2008 Feb;9(2):152-69. Curr Opin Investig Drugs. 2008. PMID: 18246518 Review.
-
Current Concepts for the IND-Directed Development of Microbicide Products to Prevent the Sexual Transmission of HIV.Curr Top Med Chem. 2016;16(10):1118-34. doi: 10.2174/1568026615666150901113939. Curr Top Med Chem. 2016. PMID: 26324047 Review.
Cited by
-
Chemically Modified Human Serum Albumin Potently Blocks Entry of Ebola Pseudoviruses and Viruslike Particles.Antimicrob Agents Chemother. 2017 Mar 24;61(4):e02168-16. doi: 10.1128/AAC.02168-16. Print 2017 Apr. Antimicrob Agents Chemother. 2017. PMID: 28167539 Free PMC article.
-
Polyanionic candidate microbicides accelerate the formation of semen-derived amyloid fibrils to enhance HIV-1 infection.PLoS One. 2013;8(3):e59777. doi: 10.1371/journal.pone.0059777. Epub 2013 Mar 27. PLoS One. 2013. PMID: 23544097 Free PMC article.
-
A bivalent recombinant protein inactivates HIV-1 by targeting the gp41 prehairpin fusion intermediate induced by CD4 D1D2 domains.Retrovirology. 2012 Dec 7;9:104. doi: 10.1186/1742-4690-9-104. Retrovirology. 2012. PMID: 23217195 Free PMC article.
-
Combinations of 3-hydroxyphthalic anhydride-modified ovalbumin with antiretroviral drug-based microbicide candidates display synergistic and complementary effects against HIV-1 infection.J Acquir Immune Defic Syndr. 2011 Apr 15;56(5):384-92. doi: 10.1097/QAI.0b013e31820a4a8d. J Acquir Immune Defic Syndr. 2011. PMID: 21239999 Free PMC article.
-
Tolcapone Potently Inhibits Seminal Amyloid Fibrils Formation and Blocks Entry of Ebola Pseudoviruses.Front Microbiol. 2020 Apr 30;11:504. doi: 10.3389/fmicb.2020.00504. eCollection 2020. Front Microbiol. 2020. PMID: 32425892 Free PMC article.
References
-
- UNAIDS. 2008 Report on the global AIDS epidemic. 2008. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_...
-
- Pauwels R, De Clercq E. Development of vaginal microbicides for the prevention of heterosexual transmission of HIV. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:211–221. - PubMed
-
- Geuenich S, Goffinet C, Venzke S, Nolkemper S, Baumann I, Plinkert P, Reichling J, Keppler OT. Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-HIV-1 activity by increasing the virion density. Retrovirology. 2008;5:27. doi: 10.1186/1742-4690-5-27. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

