CCL3 and CCL20-recruited dendritic cells modified by melanoma antigen gene-1 induce anti-tumor immunity against gastric cancer ex vivo and in vivo
- PMID: 20420712
- PMCID: PMC2873423
- DOI: 10.1186/1756-9966-29-37
CCL3 and CCL20-recruited dendritic cells modified by melanoma antigen gene-1 induce anti-tumor immunity against gastric cancer ex vivo and in vivo
Abstract
Background: To investigate whether dendritic cell (DC) precursors, recruited by injection of chemokine ligand 3 (CCL3) and CCL20, induce anti-tumor immunity against gastric cancer induced by a DC vaccine expressing melanoma antigen gene-1 (MAGE-1) ex vivo and in vivo.
Methods: B6 mice were injected with CCL3 and CCL20 via the tail vein. Freshly isolated F4/80-B220-CD11c+ cells cultured with cytokines were analyzed by phenotype analysis and mixed lymphocyte reaction (MLR). For adenoviral (Ad)-mediated gene transduction, cultured F4/80-B220-CD11c+ cells were incubated with Ad-MAGE-1. Vaccination of stimulated DC induced T lymphocytes. The killing effect of these T cells against gastric carcinoma cells was assayed by MTT. INF-gamma production was determined with an INF-gamma ELISA kit. In the solid tumor and metastases model, DC-based vaccines were used for immunization after challenge with MFC cells. Tumor size, survival of mice, and number of pulmonary metastatic foci were used to assess the therapeutic effect of DC vaccines.
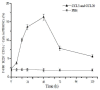
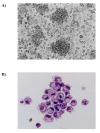
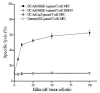
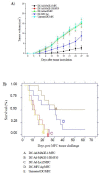
Results: F4/80-B220-CD11c+ cell numbers increased after CCL3 and CCL20 injection. Freshly isolated F4/80-B220-CD11c+ cells cultured with cytokines were phenotyically identical to typical DC and gained the capacity to stimulate allogeneic T cells. These DCs were transduced with Ad-MAGE-1, which were prepared for DC vaccines expressing tumor antigen. T lymphocytes stimulated by DCs transduced with Ad-MAGE-1 exhibited specific killing effects on gastric carcinoma cells and produced high levels of INF-gamma ex vivo. In vivo, tumor sizes of the experimental group were much smaller than both the positive control group and the negative control groups (P < 0.05). Kaplan-Meier survival curves showed that survival of the experimental group mice was significantly longer than the control groups (P < 0.05). In addition, MAGE-1-transduced DCs were also a therapeutic benefit on an established metastatic tumor, resulting in a tremendous decrease in the number of pulmonary metastatic foci.
Conclusions: CCL3 and CCL20-recruited DCs modified by adenovirus-trasnsduced, tumor-associated antigen, MAGE-1, can stimulate anti-tumor immunity specific to gastric cancer ex vivo and in vivo. This system may prove to be an efficient strategy for anti-tumor immunotherapy.
Figures



References
-
- Guida F, Formisano G, Esposito D, Antonino A, Conte P, Bencivenga M, Persico M, Avallone U. Gastric cancer: surgical treatment and prognostic score. Minerva Chir. 2008;63:93–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

