Primary prevention of colorectal cancer
- PMID: 20420944
- PMCID: PMC2947820
- DOI: 10.1053/j.gastro.2010.01.057
Primary prevention of colorectal cancer
Abstract
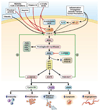
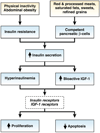
Colorectal cancer has been strongly associated with a Western lifestyle. In the past several decades, much has been learned about the dietary, lifestyle, and medication risk factors for this malignancy. Although there is controversy about the role of specific nutritional factors, consideration of dietary pattern as a whole appears useful for formulating recommendations. For example, several studies have shown that high intake of red and processed meats, highly refined grains and starches, and sugars is related to increased risk of colorectal cancer. Replacing these factors with poultry, fish, and plant sources as the primary source of protein; unsaturated fats as the primary source of fat; and unrefined grains, legumes and fruits as the primary source of carbohydrates is likely to lower risk of colorectal cancer. Although a role for supplements, including vitamin D, folate, and vitamin B6, remains uncertain, calcium supplementation is likely to be at least modestly beneficial. With respect to lifestyle, compelling evidence indicates that avoidance of smoking and heavy alcohol use, prevention of weight gain, and maintenance of a reasonable level of physical activity are associated with markedly lower risks of colorectal cancer. Medications such as aspirin and nonsteroidal anti-inflammatory drugs and postmenopausal hormones for women are associated with substantial reductions in colorectal cancer risk, though their utility is affected by associated risks. Taken together, modifications in diet and lifestyle should substantially reduce the risk of colorectal cancer and could complement screening in reducing colorectal cancer incidence.
Conflict of interest statement
Financial Disclosures: No conflicts of interest exist.
Figures



References
-
- American Cancer Society. Cancer Facts and Figures 2009. Atlanta: American Cancer Society; 2009.
-
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. IARC Scientific Publications No. 160. Vol. IX. Lyon: IARC; 2007. Cancer incidence in five continents.
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Giovannucci E, Willett WC. Dietary factors and risk of colon cancer. Ann Med. 1994;26:443–452. - PubMed
-
- Burkitt DP. Related disease--related cause? Lancet. 1969;2:1229–1231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

