The chromosomal instability pathway in colon cancer
- PMID: 20420946
- PMCID: PMC4243705
- DOI: 10.1053/j.gastro.2009.12.065
The chromosomal instability pathway in colon cancer
Abstract
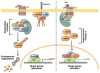
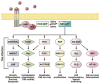
The acquisition of genomic instability is a crucial feature in tumor development and there are at least 3 distinct pathways in colorectal cancer pathogenesis: the chromosomal instability (CIN), microsatellite instability, and CpG island methylator phenotype pathways. Most cases of colorectal cancer arise through the CIN pathway, which is characterized by widespread imbalances in chromosome number (aneuploidy) and loss of heterozygosity. It can result from defects in chromosomal segregation, telomere stability, and the DNA damage response, although the full complement of genes underlying CIN remains incompletely described. Coupled with the karyotypic abnormalities observed in CIN tumors are the accumulation of a characteristic set of mutations in specific tumor suppressor genes and oncogenes that activate pathways critical for colorectal cancer initiation and progression. Whether CIN creates the appropriate milieu for the accumulation of these mutations or vice versa remains a provocative and unanswered question. The goal of this review is to provide an updated perspective on the mechanisms that lead to CIN and the key mutations that are acquired in this pathway.
Figures


References
-
- Fearon E, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. - PubMed
-
- Markowitz S, Wang J, Myeroff L, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. - PubMed
-
- Thiagalingam S, Lengauer C, Leach FS, et al. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–346. - PubMed
-
- Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3:1221–1224. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

