Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer
- PMID: 20421152
- PMCID: PMC3001108
- DOI: 10.1016/j.ijrobp.2010.02.020
Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer
Abstract
Purpose: Protection of bone marrow against radiotoxicity during radioimmunotherapy and in some cases external beam radiation therapy such as hemi-body irradiation would permit administration of significantly higher doses to tumors, resulting in increased efficacy and safety of treatment. Melanin, a naturally occurring pigment, possesses radioprotective properties. We hypothesized that melanin, which is insoluble, could be delivered to the bone marrow by intravenously administrated melanin-covered nanoparticles (MNs) because of the human body's "self-sieving" ability, protecting it against ionizing radiation.
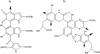
Methods and materials: The synthesis of MNs was performed via enzymatic polymerization of 3,4-dihydroxyphenylalanine and/or 5-S-cysteinyl-3,4-dihydroxyphenylalanine on the surface of 20-nm plain silica nanoparticles. The biodistribution of radiolabeled MNs in mice was done at 3 and 24 h. Healthy CD-1 mice (Charles River Laboratories International, Inc., Wilmington, MA) or melanoma tumor-bearing nude mice were given MNs intravenously, 50 mg/kg of body weight, 3 h before either whole-body exposure to 125 cGy or treatment with 1 mCi of (188)Re-labeled 6D2 melanin-binding antibody.
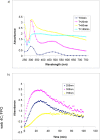
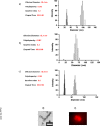
Results: Polymerization of melanin precursors on the surface of silica nanoparticles resulted in formation of a 15-nm-thick melanin layer as confirmed by light scattering, transmission electron microscopy, and immunofluorescence. The biodistribution after intravenous administration showed than MN uptake in bone marrow was 0.3% and 0.2% of injected dose per gram at 3 and 24 h, respectively, whereas pre-injection with pluronic acid increased the uptake to 6% and 3% of injected dose per gram, respectively. Systemic MN administration reduced hematologic toxicity in mice treated with external radiation or radioimmunotherapy, whereas no tumor protection by MNs was observed.
Conclusions: MNs or similar structures provide a novel approach to protection of bone marrow from ionizing radiation based on prevention of free radical formation by melanin.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Melanin nanoparticles (MNPs) provide protection against whole-body ɣ-irradiation in mice via restoration of hematopoietic tissues.Mol Cell Biochem. 2015 Jan;399(1-2):59-69. doi: 10.1007/s11010-014-2232-y. Epub 2014 Oct 10. Mol Cell Biochem. 2015. PMID: 25300618
-
p53-Based Strategy for Protection of Bone Marrow From Y-90 Ibritumomab Tiuxetan.Int J Radiat Oncol Biol Phys. 2015 Aug 1;92(5):1116-1122. doi: 10.1016/j.ijrobp.2015.04.003. Epub 2015 Apr 8. Int J Radiat Oncol Biol Phys. 2015. PMID: 26025778 Free PMC article.
-
Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a mAb to melanin.Proc Natl Acad Sci U S A. 2004 Oct 12;101(41):14865-70. doi: 10.1073/pnas.0406180101. Epub 2004 Oct 5. Proc Natl Acad Sci U S A. 2004. PMID: 15469923 Free PMC article.
-
Radioimmunotherapy of experimental human metastatic melanoma with melanin-binding antibodies and in combination with dacarbazine.Clin Cancer Res. 2009 Apr 1;15(7):2373-9. doi: 10.1158/1078-0432.CCR-08-2376. Epub 2009 Mar 17. Clin Cancer Res. 2009. PMID: 19293257
-
Pre-clinical evaluation and efficacy studies of a melanin-binding IgM antibody labeled with 188Re against experimental human metastatic melanoma in nude mice.Cancer Biol Ther. 2008 Jul;7(7):1116-27. doi: 10.4161/cbt.7.7.6197. Epub 2008 Apr 28. Cancer Biol Ther. 2008. PMID: 18535406
Cited by
-
Melanin nanoparticles as a novel contrast agent for optoacoustic tomography.Photoacoustics. 2015 Feb 14;3(1):35-43. doi: 10.1016/j.pacs.2015.02.001. eCollection 2015 Mar. Photoacoustics. 2015. PMID: 25893172 Free PMC article.
-
Radiotherapy and immune response: the systemic effects of a local treatment.Clinics (Sao Paulo). 2018 Dec 10;73(suppl 1):e557s. doi: 10.6061/clinics/2018/e557s. Clinics (Sao Paulo). 2018. PMID: 30540123 Free PMC article. Review.
-
Space exploration as a catalyst for medical innovations.Front Med (Lausanne). 2023 Jul 19;10:1226531. doi: 10.3389/fmed.2023.1226531. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37538310 Free PMC article.
-
Convergence of nanotechnology with radiation therapy-insights and implications for clinical translation.Transl Cancer Res. 2013 Aug 23;2(4):256-268. doi: 10.3978/j.issn.2218-676X.2013.08.10. Transl Cancer Res. 2013. PMID: 25279336 Free PMC article.
-
Nanodrugs with intrinsic radioprotective exertion: Turning the double-edged sword into a single-edged knife.Exploration (Beijing). 2023 Mar 31;3(2):20220119. doi: 10.1002/EXP.20220119. eCollection 2023 Apr. Exploration (Beijing). 2023. PMID: 37324033 Free PMC article. Review.
References
-
- Hill HZ. The function of melanin or six blind people examine an elephant. Bioessays. 1992;14:49–56. - PubMed
-
- Nosanchuk JD, Casadevall A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003;5:203–223. - PubMed
-
- Robinson CH. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001;151:341–353.
-
- Mironenko NV, Alekhina IA, Zhdanova NN, et al. Intraspecific variation in gamma-radiation resistance and genomic structure in the filamentous fungus Alternaria alternata: A case study of strains inhabiting Chernobyl reactor no. 4. Ecotoxicol Environ Saf. 2000;45:177–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

