Deregulated Cdc6 inhibits DNA replication and suppresses Cdc7-mediated phosphorylation of Mcm2-7 complex
- PMID: 20421204
- PMCID: PMC2938227
- DOI: 10.1093/nar/gkq262
Deregulated Cdc6 inhibits DNA replication and suppresses Cdc7-mediated phosphorylation of Mcm2-7 complex
Abstract
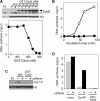
Mcm2-7 is recruited to eukaryotic origins of DNA replication by origin recognition complex, Cdc6 and Cdt1 thereby licensing the origins. Cdc6 is essential for origin licensing during DNA replication and is readily destabilized from chromatin after Mcm2-7 loading. Here, we show that after origin licensing, deregulation of Cdc6 suppresses DNA replication in Xenopus egg extracts without the involvement of ATM/ATR-dependent checkpoint pathways. DNA replication is arrested specifically after chromatin binding of Cdc7, but before Cdk2-dependent pathways and deregulating Cdc6 after this step does not impair activation of origin firing or elongation. Detailed analyses revealed that Cdc6 deregulation leads to strong suppression of Cdc7-mediated hyperphosphorylation of Mcm4 and subsequent chromatin loading of Cdc45, Sld5 and DNA polymerase α. Mcm2 phosphorylation is also repressed although to a lesser extent. Remarkably, Cdc6 itself does not directly inhibit Cdc7 kinase activity towards Mcm2-4-6-7 in purified systems, rather modulates Mcm2-7 phosphorylation on chromatin context. Taken together, we propose that Cdc6 on chromatin acts as a modulator of Cdc7-mediated phosphorylation of Mcm2-7, and thus destabilization of Cdc6 from chromatin after licensing is a key event ensuring proper transition to the initiation of DNA replication.
Figures





Similar articles
-
MCM2-7 complexes bind chromatin in a distributed pattern surrounding the origin recognition complex in Xenopus egg extracts.J Biol Chem. 2002 Sep 6;277(36):33049-57. doi: 10.1074/jbc.M204438200. Epub 2002 Jun 26. J Biol Chem. 2002. PMID: 12087101
-
MCM interference during licensing of DNA replication in Xenopus egg extracts-Possible Role of a C-terminal region of MCM3.Cell Cycle. 2018;17(4):492-505. doi: 10.1080/15384101.2017.1415681. Epub 2018 Jan 30. Cell Cycle. 2018. PMID: 29261034 Free PMC article.
-
Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing.Cell. 2009 Nov 13;139(4):719-30. doi: 10.1016/j.cell.2009.10.015. Epub 2009 Nov 5. Cell. 2009. PMID: 19896182 Free PMC article.
-
Behavior of replication origins in Eukaryota - spatio-temporal dynamics of licensing and firing.Cell Cycle. 2015;14(14):2251-64. doi: 10.1080/15384101.2015.1056421. Epub 2015 Jun 1. Cell Cycle. 2015. PMID: 26030591 Free PMC article. Review.
-
Initiating DNA synthesis: from recruiting to activating the MCM complex.J Cell Sci. 2001 Apr;114(Pt 8):1447-54. doi: 10.1242/jcs.114.8.1447. J Cell Sci. 2001. PMID: 11282021 Review.
Cited by
-
A nontranscriptional role for HIF-1α as a direct inhibitor of DNA replication.Sci Signal. 2013 Feb 12;6(262):ra10. doi: 10.1126/scisignal.2003417. Sci Signal. 2013. PMID: 23405012 Free PMC article.
-
Characterization of an archaeal virus-host system reveals massive genomic rearrangements in a laboratory strain.Front Microbiol. 2023 Sep 18;14:1274068. doi: 10.3389/fmicb.2023.1274068. eCollection 2023. Front Microbiol. 2023. PMID: 37789858 Free PMC article.
-
Molecular pathology of cutaneous melanoma.Melanoma Manag. 2014 Nov;1(2):151-164. doi: 10.2217/mmt.14.23. Epub 2014 Dec 4. Melanoma Manag. 2014. PMID: 30190820 Free PMC article. Review.
-
Reconstruction of pathway modification induced by nicotinamide using multi-omic network analyses in triple negative breast cancer.Sci Rep. 2017 Jun 14;7(1):3466. doi: 10.1038/s41598-017-03322-7. Sci Rep. 2017. PMID: 28615672 Free PMC article.
-
Biphasic chromatin binding of histone chaperone FACT during eukaryotic chromatin DNA replication.Biochim Biophys Acta. 2011 Jun;1813(6):1129-36. doi: 10.1016/j.bbamcr.2011.01.002. Epub 2011 Jan 11. Biochim Biophys Acta. 2011. PMID: 21232560 Free PMC article.
References
-
- Coleman TR, Carpenter PB, Dunphy WG. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. - PubMed
-
- Rowles A, Chong JP, Brown L, Howell M, Evan GI, Blow JJ. Interaction between the origin recognition complex and the replication licensing system in Xenopus. Cell. 1996;87:287–296. - PubMed
-
- Takahashi TS, Wigley DB, Walter JC. Pumps, paradoxes and ploughshares: mechanism of the MCM2–7 DNA helicase. Trends Biochem. Sci. 2005;30:437–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

