PIAS1 regulates CP2c localization and active promoter complex formation in erythroid cell-specific alpha-globin expression
- PMID: 20421208
- PMCID: PMC2938217
- DOI: 10.1093/nar/gkq286
PIAS1 regulates CP2c localization and active promoter complex formation in erythroid cell-specific alpha-globin expression
Abstract
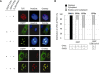
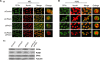
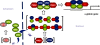
Data presented here extends our previous observations on α-globin transcriptional regulation by the CP2 and PIAS1 proteins. Using RNAi knockdown, we have now shown that CP2b, CP2c and PIAS1 are each necessary for synergistic activation of endogenous α-globin gene expression in differentiating MEL cells. In this system, truncated PIAS1 mutants lacking the ring finger domain recruited CP2c to the nucleus, as did wild-type PIAS1, demonstrating that this is a sumoylation-independent process. In vitro, recombinant CP2c, CP2b and PIAS1 bound DNA as a stable CBP (CP2c/CP2b/PIAS1) complex. Following PIAS1 knockdown in MEL cells, however, the association of endogenous CP2c and CP2b with the α-globin promoter simultaneously decreased. By mapping the CP2b- and CP2c-binding domains on PIAS1, and the PIAS1-binding domains on CP2b and CP2c, we found that two regions of PIAS1 that interact with CP2c/CP2b are required for its co-activator function. We propose that CP2c, CP2b, and PIAS1 form a hexametric complex with two units each of CP2c, CP2b, and PIAS1, in which PIAS1 serves as a clamp between two CP2 proteins, while CP2c binds directly to the target DNA and CP2b mediates strong transactivation.
Figures






Similar articles
-
Structural and Functional Insights into CP2c Transcription Factor Complexes.Int J Mol Sci. 2022 Jun 7;23(12):6369. doi: 10.3390/ijms23126369. Int J Mol Sci. 2022. PMID: 35742810 Free PMC article.
-
The relative cellular levels of CP2a and CP2b potentiates erythroid cell-specific expression of the alpha-globin gene by regulating the nuclear localization of CP2c.Biochem Biophys Res Commun. 2009 Mar 20;380(4):813-7. doi: 10.1016/j.bbrc.2009.01.172. Epub 2009 Feb 4. Biochem Biophys Res Commun. 2009. PMID: 19338758
-
Erythroid cell-specific alpha-globin gene regulation by the CP2 transcription factor family.Mol Cell Biol. 2005 Jul;25(14):6005-20. doi: 10.1128/MCB.25.14.6005-6020.2005. Mol Cell Biol. 2005. PMID: 15988015 Free PMC article.
-
Mbd2-CP2c loop drives adult-type globin gene expression and definitive erythropoiesis.Nucleic Acids Res. 2018 Jun 1;46(10):4933-4949. doi: 10.1093/nar/gky193. Nucleic Acids Res. 2018. PMID: 29547954 Free PMC article.
-
Protein inhibitors of activated STAT (Pias1 and Piasy) differentially regulate pituitary homeobox 2 (PITX2) transcriptional activity.J Biol Chem. 2013 May 3;288(18):12580-95. doi: 10.1074/jbc.M112.374561. Epub 2013 Mar 20. J Biol Chem. 2013. PMID: 23515314 Free PMC article.
Cited by
-
Rational discovery of antimetastatic agents targeting the intrinsically disordered region of MBD2.Sci Adv. 2019 Nov 20;5(11):eaav9810. doi: 10.1126/sciadv.aav9810. eCollection 2019 Nov. Sci Adv. 2019. PMID: 31799386 Free PMC article.
-
SUMOylation-mediated PSME3-20S proteasomal degradation of transcription factor CP2c is crucial for cell cycle progression.Sci Adv. 2023 Jan 27;9(4):eadd4969. doi: 10.1126/sciadv.add4969. Epub 2023 Jan 27. Sci Adv. 2023. PMID: 36706181 Free PMC article.
-
Structural and Functional Insights into CP2c Transcription Factor Complexes.Int J Mol Sci. 2022 Jun 7;23(12):6369. doi: 10.3390/ijms23126369. Int J Mol Sci. 2022. PMID: 35742810 Free PMC article.
-
A Cell-Penetrant Peptide Disrupting the Transcription Factor CP2c Complexes Induces Cancer-Specific Synthetic Lethality.Adv Sci (Weinh). 2023 Nov;10(33):e2305096. doi: 10.1002/advs.202305096. Epub 2023 Oct 16. Adv Sci (Weinh). 2023. PMID: 37845006 Free PMC article.
-
Diagnostic and prognostic relevance of CP2c and YY1 expression in hepatocellular carcinoma.Oncotarget. 2017 Apr 11;8(15):24389-24400. doi: 10.18632/oncotarget.15462. Oncotarget. 2017. PMID: 28412749 Free PMC article.
References
-
- Zhou W, Zhao Q, Sutton R, Cumming H, Wang X, Cerruti L, Hall M, Wu R, Cunningham JM, Jane SM. The role of p22 NF-E4 in human globin gene switching. J. Biol. Chem. 2004;279:26227–26232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

