Direct endosomal acidification by the outwardly rectifying CLC-5 Cl(-)/H(+) exchanger
- PMID: 20421284
- PMCID: PMC2911210
- DOI: 10.1113/jphysiol.2010.188540
Direct endosomal acidification by the outwardly rectifying CLC-5 Cl(-)/H(+) exchanger
Abstract
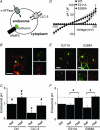
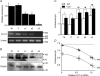
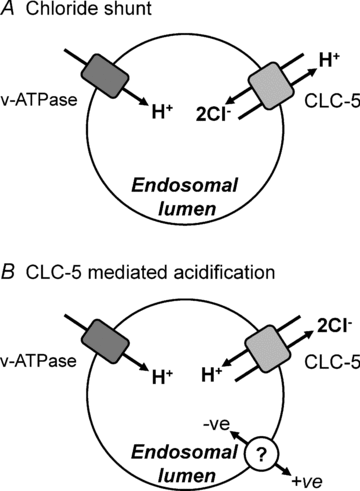
The voltage-gated Cl(-) channel (CLC) family comprises cell surface Cl(-) channels and intracellular Cl(-)/H(+) exchangers. CLCs in organelle membranes are thought to assist acidification by providing a passive chloride conductance that electrically counterbalances H(+) accumulation. Following recent descriptions of Cl(-)/H(+) exchange activity in endosomal CLCs we have re-evaluated their role. We expressed human CLC-5 in HEK293 cells, recorded currents under a range of Cl(-) and H(+) gradients by whole-cell patch clamp, and examined the contribution of CLC-5 to endosomal acidification using a targeted pH-sensitive fluorescent protein. We found that CLC-5 only conducted outward currents, corresponding to Cl(-) flux into the cytoplasm and H(+) from the cytoplasm. Inward currents were never observed, despite the range of intracellular and extracellular Cl(-) concentrations and pH used. Endosomal acidification in HEK293 cells was prevented by 25 microm bafilomycin-A1, an inhibitor of vacuolar-type H(+)-ATPase (v-ATPase), which actively pumps H(+) into the endosomal lumen. Overexpression of CLC-5 in HEK293 cells conferred an additional bafilomycin-insensitive component to endosomal acidification. This effect was abolished by making mutations in CLC-5 that remove H(+) transport, which result in either no current (E268A) or bidirectional Cl(-) flux (E211A). Endosomal acidification in a proximal tubule cell line was partially sensitive to inhibition of v-ATPase by bafilomycin-A1. Furthermore, in the presence of bafilomycin-A1, acidification was significantly reduced and nearly fully ablated by partial and near-complete knockdown of endogenous CLC-5 by siRNA. These data suggest that CLC-5 is directly involved in endosomal acidification by exchanging endosomal Cl(-) for H(+).
Figures




Similar articles
-
A pure chloride channel mutant of CLC-5 causes Dent's disease via insufficient V-ATPase activation.Pflugers Arch. 2016 Jul;468(7):1183-1196. doi: 10.1007/s00424-016-1808-7. Epub 2016 Apr 5. Pflugers Arch. 2016. PMID: 27044412
-
ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation.J Biol Chem. 2005 Jan 14;280(2):1241-7. doi: 10.1074/jbc.M407030200. Epub 2004 Oct 25. J Biol Chem. 2005. PMID: 15504734
-
Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins.Nature. 2005 Jul 21;436(7049):424-7. doi: 10.1038/nature03860. Nature. 2005. PMID: 16034422
-
Cell biology and physiology of CLC chloride channels and transporters.Compr Physiol. 2012 Jul;2(3):1701-44. doi: 10.1002/cphy.c110038. Compr Physiol. 2012. PMID: 23723021 Review.
-
ClC-5: Physiological role and biophysical mechanisms.Cell Calcium. 2015 Jul;58(1):57-66. doi: 10.1016/j.ceca.2014.09.007. Epub 2014 Nov 13. Cell Calcium. 2015. PMID: 25443653 Review.
Cited by
-
Chloride Channel-3 (ClC-3) Modifies the Trafficking of Leucine-Rich Repeat-Containing 8A (LRRC8A) Anion Channels.J Membr Biol. 2023 Apr;256(2):125-135. doi: 10.1007/s00232-022-00271-9. Epub 2022 Nov 2. J Membr Biol. 2023. PMID: 36322172 Free PMC article.
-
Dent disease: A window into calcium and phosphate transport.J Cell Mol Med. 2019 Nov;23(11):7132-7142. doi: 10.1111/jcmm.14590. Epub 2019 Aug 31. J Cell Mol Med. 2019. PMID: 31472005 Free PMC article. Review.
-
Census of halide-binding sites in protein structures.Bioinformatics. 2020 May 1;36(10):3064-3071. doi: 10.1093/bioinformatics/btaa079. Bioinformatics. 2020. PMID: 32022861 Free PMC article.
-
TNFα and Reactive Oxygen Signaling in Vascular Smooth Muscle Cells in Hypertension and Atherosclerosis.Am J Hypertens. 2020 Oct 21;33(10):902-913. doi: 10.1093/ajh/hpaa089. Am J Hypertens. 2020. PMID: 32498083 Free PMC article. Review.
-
The Site and Type of CLCN5 Genetic Variation Impact the Resulting Dent Disease-1 Phenotype.Kidney Int Rep. 2023 Mar 23;8(6):1220-1230. doi: 10.1016/j.ekir.2023.03.012. eCollection 2023 Jun. Kidney Int Rep. 2023. PMID: 37284679 Free PMC article.
References
-
- Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of CLC Cl− channels. Nature. 2004;427:803–807. - PubMed
-
- Devuyst O, Christie PT, Courtoy PJ, Beauwens R, Thakker RV. Intra-renal and subcellular distribution of the human chloride channel, CLC-5, reveals a pathophysiological basis for Dent's disease. Hum Mol Genet. 1999;8:247–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

