Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides
- PMID: 20421285
- PMCID: PMC2911224
- DOI: 10.1113/jphysiol.2009.186643
Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides
Abstract
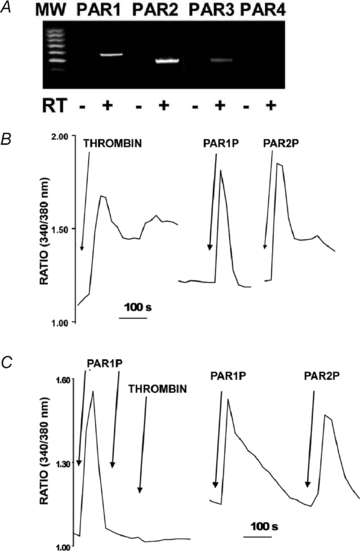
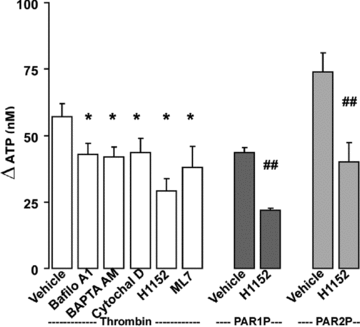
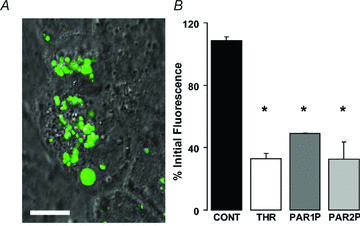
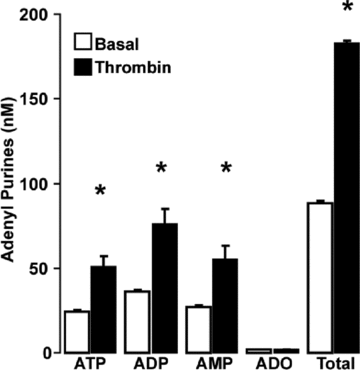
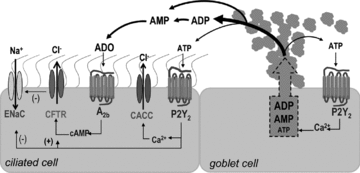
Purinergic regulation of airway innate defence activities is in part achieved by the release of nucleotides from epithelial cells. However, the mechanisms of airway epithelial nucleotide release are poorly understood. We have previously demonstrated that ATP is released from ionomycin-stimulated airway epithelial goblet cells coordinately with mucin exocytosis, suggesting that ATP is released as a co-cargo molecule from mucin-containing granules. We now demonstrate that protease-activated-receptor (PAR) agonists also stimulate the simultaneous release of mucins and ATP from airway epithelial cells. PAR-mediated mucin and ATP release were dependent on intracellular Ca(2+) and actin cytoskeleton reorganization since BAPTA AM, cytochalasin D, and inhibitors of Rho and myosin light chain kinases blocked both responses. To test the hypothesis that ATP is co-released with mucin from mucin granules, we measured the nucleotide composition of isolated mucin granules purified based on their MUC5AC and VAMP-8 content by density gradients. Mucin granules contained ATP, but the levels of ADP and AMP within granules exceeded by nearly 10-fold that of ATP. Consistent with this finding, apical secretions from PAR-stimulated cells contained relatively high levels of ADP/AMP, which could not be accounted for solely based on ATP release and hydrolysis. Thus, mucin granules contribute to ATP release and also are a source of extracellular ADP and AMP. Direct release of ADP/AMP from mucin granules is likely to provide a major source of airway surface adenosine to signal in a paracrine faction ciliated cell A(2b) receptors to activate ion/water secretion and appropriately hydrate goblet cell-released mucins.
Figures


Similar articles
-
Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules.Am J Physiol Cell Physiol. 2013 May 15;304(10):C976-84. doi: 10.1152/ajpcell.00371.2012. Epub 2013 Mar 6. Am J Physiol Cell Physiol. 2013. PMID: 23467297 Free PMC article.
-
Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells.J Physiol. 2007 Oct 1;584(Pt 1):245-59. doi: 10.1113/jphysiol.2007.139840. Epub 2007 Jul 26. J Physiol. 2007. PMID: 17656429 Free PMC article.
-
Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium.Am J Respir Cell Mol Biol. 2011 Aug;45(2):253-60. doi: 10.1165/rcmb.2010-0253OC. Epub 2010 Oct 8. Am J Respir Cell Mol Biol. 2011. PMID: 20935191 Free PMC article.
-
Nucleotide release by airway epithelia.Subcell Biochem. 2011;55:1-15. doi: 10.1007/978-94-007-1217-1_1. Subcell Biochem. 2011. PMID: 21560042 Review.
-
Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling.Respir Physiol Neurobiol. 2008 Nov 30;163(1-3):208-13. doi: 10.1016/j.resp.2008.05.015. Epub 2008 May 28. Respir Physiol Neurobiol. 2008. PMID: 18635403 Free PMC article. Review.
Cited by
-
Neutrophil elastase induces MUC5AC secretion via protease-activated receptor 2.Mol Cell Biochem. 2013 May;377(1-2):75-85. doi: 10.1007/s11010-013-1572-3. Epub 2013 Feb 8. Mol Cell Biochem. 2013. PMID: 23392769
-
Airway Epithelial Nucleotide Release Contributes to Mucociliary Clearance.Life (Basel). 2021 May 11;11(5):430. doi: 10.3390/life11050430. Life (Basel). 2021. PMID: 34064654 Free PMC article. Review.
-
Allergic sensitization enhances anion current responsiveness of murine trachea to PAR-2 activation.Pflugers Arch. 2012 Mar;463(3):497-509. doi: 10.1007/s00424-011-1064-9. Epub 2011 Dec 15. Pflugers Arch. 2012. PMID: 22170096
-
Purinergic receptors in airway hydration.Biochem Pharmacol. 2021 May;187:114387. doi: 10.1016/j.bcp.2020.114387. Epub 2021 Jan 5. Biochem Pharmacol. 2021. PMID: 33358825 Free PMC article. Review.
-
Inflammation promotes airway epithelial ATP release via calcium-dependent vesicular pathways.Am J Respir Cell Mol Biol. 2013 Nov;49(5):814-20. doi: 10.1165/rcmb.2012-0493OC. Am J Respir Cell Mol Biol. 2013. PMID: 23763446 Free PMC article.
References
-
- Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–17138. - PubMed
-
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. - PubMed
-
- Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. - PubMed
-
- Davis CW, Dowell ML, Lethem MI, Van Scott M. Goblet cell degranulation in isolated canine tracheal epithelium: Response to exogenous ATP, ADP, and adenosine. Am J Physiol Cell Physiol. 1992;262:C1313–C1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

