Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux
- PMID: 20421331
- PMCID: PMC2912048
- DOI: 10.1124/jpet.110.167601
Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux
Abstract
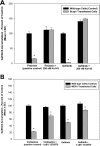
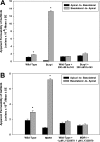
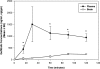
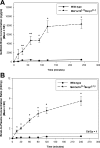
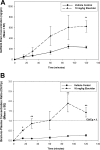
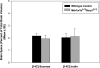
Gefitinib is an orally active inhibitor of the epidermal growth factor receptor approved for use in patients with locally advanced or metastatic non-small cell lung cancer. It has also been evaluated in several clinical trials for treatment of brain tumors such as high-grade glioma. In this study, we investigated the influence of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) on distribution of gefitinib to the central nervous system. In vitro studies conducted in Madin-Darby canine kidney II cells indicate that both P-gp and BCRP effectively transport gefitinib, limiting its intracellular accumulation. In vivo studies demonstrated that transport of gefitinib across the blood-brain barrier (BBB) is significantly limited. Steady-state brain-to-plasma (B/P) concentration ratios were 70-fold higher in the Mdr1a/b(-/-) Bcrp1(-/-) mice (ratio of approximately 7) compared with wild-type mice (ratio of approximately 0.1). The B/P ratio after oral administration increased significantly when gefitinib was coadministered with the dual P-gp and BCRP inhibitor elacridar. We investigated the integrity of tight junctions in the Mdr1a/b(-/-) Bcrp1(-/-) mice and found no difference in the brain inulin and sucrose space between the wild-type and Mdr1a/b(-/-) Bcrp1(-/-) mice. This suggested that the dramatic enhancement in the brain distribution of gefitinib is not due to a leakier BBB in these mice. These results show that brain distribution of gefitinib is restricted due to active efflux by P-gp and BCRP. This finding is of clinical significance for therapy in brain tumors such as glioma, where concurrent administration of a dual inhibitor such as elacridar can increase delivery and thus enhance efficacy of gefitinib.
Figures

References
-
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 1:417–425 - PubMed
-
- Arteaga CL, Johnson DH. (2001) Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr Opin Oncol 13:491–498 - PubMed
-
- Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JGNew Approaches to Brain Tumor Therapy (NABTT) Consortium (2009) Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol 91:51–58 - PMC - PubMed
-
- Brandes AA, Franceschi E, Tosoni A, Hegi ME, Stupp R. (2008) Epidermal growth factor receptor inhibitors in neuro-oncology: hopes and disappointments. Clin Cancer Res 14:957–960 - PubMed
-
- CBTRUS (2008) CBTRUS 2008 Statistical Report: Primary Brain Tumors in the United States, 2000–2004, Central Brain Tumor Registry of the United States, Hinsdale, IL
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

