Coupling and cooperativity in voltage activation of a limited-state BK channel gating in saturating Ca2+
- PMID: 20421372
- PMCID: PMC2860587
- DOI: 10.1085/jgp.200910331
Coupling and cooperativity in voltage activation of a limited-state BK channel gating in saturating Ca2+
Abstract
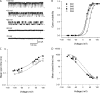
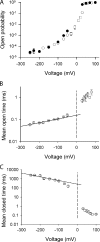
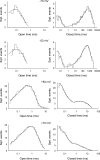
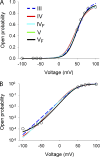
Voltage-dependent gating mechanisms of large conductance Ca(2+) and voltage-activated (BK) channels were investigated using two-dimensional maximum likelihood analysis of single-channel open and closed intervals. To obtain sufficient data at negative as well as positive voltages, single-channel currents were recorded at saturating Ca(2+) from BK channels mutated to remove the RCK1 Ca(2+) and Mg(2+) sensors. The saturating Ca(2+) acting on the Ca(2+) bowl sensors of the resulting BK(B) channels increased channel activity while driving the gating into a reduced number of states, simplifying the model. Five highly constrained idealized gating mechanisms based on extensions of the Monod-Wyman-Changeux model for allosteric proteins were examined. A 10-state model without coupling between the voltage sensors and the opening/closing transitions partially described the voltage dependence of Po but not the single-channel kinetics. With allowed coupling, the model gave improved descriptions of Po and approximated the single-channel kinetics; each activated voltage sensor increased the opening rate approximately an additional 23-fold while having little effect on the closing rate. Allowing cooperativity among voltage sensors further improved the description of the data: each activated voltage sensor increased the activation rate of the remaining voltage sensors approximately fourfold, with little effect on the deactivation rate. The coupling factor was decreased in models with cooperativity from approximately 23 to approximately 18. Whether the apparent cooperativity among voltage sensors arises from imposing highly idealized models or from actual cooperativity will require additional studies to resolve. For both cooperative and noncooperative models, allowing transitions to five additional brief (flicker) closed states further improved the description of the data. These observations show that the voltage-dependent single-channel kinetics of BK(B) channels can be approximated by highly idealized allosteric models in which voltage sensor movement increases Po mainly through an increase in channel opening rates, with limited effects on closing rates.
Figures



Similar articles
-
Intra- and intersubunit cooperativity in activation of BK channels by Ca2+.J Gen Physiol. 2006 Oct;128(4):389-404. doi: 10.1085/jgp.200609486. J Gen Physiol. 2006. PMID: 17001085 Free PMC article.
-
Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels.J Gen Physiol. 2002 Sep;120(3):267-305. doi: 10.1085/jgp.20028605. J Gen Physiol. 2002. PMID: 12198087 Free PMC article.
-
Mg2+ enhances voltage sensor/gate coupling in BK channels.J Gen Physiol. 2008 Jan;131(1):13-32. doi: 10.1085/jgp.200709877. J Gen Physiol. 2008. PMID: 18166624 Free PMC article.
-
Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage.Biol Res. 2006;39(3):385-401. doi: 10.4067/s0716-97602006000300003. Epub 2006 Nov 7. Biol Res. 2006. PMID: 17106573 Review.
-
Single-channel kinetics of BK (Slo1) channels.Front Physiol. 2015 Jan 21;5:532. doi: 10.3389/fphys.2014.00532. eCollection 2014. Front Physiol. 2015. PMID: 25653620 Free PMC article. Review.
Cited by
-
Modulation of cardiac ryanodine receptor channels by alkaline earth cations.PLoS One. 2011;6(10):e26693. doi: 10.1371/journal.pone.0026693. Epub 2011 Oct 21. PLoS One. 2011. PMID: 22039534 Free PMC article.
-
Modulation of BK channel voltage gating by different auxiliary β subunits.Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):18991-6. doi: 10.1073/pnas.1216953109. Epub 2012 Oct 29. Proc Natl Acad Sci U S A. 2012. PMID: 23112204 Free PMC article.
-
BK channel activation: structural and functional insights.Trends Neurosci. 2010 Sep;33(9):415-23. doi: 10.1016/j.tins.2010.06.004. Trends Neurosci. 2010. PMID: 20663573 Free PMC article. Review.
-
The neuropeptide GsMTx4 inhibits a mechanosensitive BK channel through the voltage-dependent modification specific to mechano-gating.J Biol Chem. 2019 Aug 2;294(31):11892-11909. doi: 10.1074/jbc.RA118.005511. Epub 2019 Jun 14. J Biol Chem. 2019. PMID: 31201274 Free PMC article.
-
Molecular mechanism of BK channel activation by the smooth muscle relaxant NS11021.J Gen Physiol. 2020 Jun 1;152(6):e201912506. doi: 10.1085/jgp.201912506. J Gen Physiol. 2020. PMID: 32221543 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

