Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages
- PMID: 20421466
- PMCID: PMC2889355
- DOI: 10.1073/pnas.0910929107
Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages
Abstract
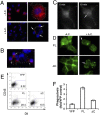
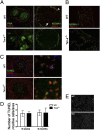
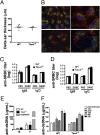
Tim-4 is a phosphatidylserine (PS) receptor that is expressed on various macrophage subsets. It mediates phagocytosis of apoptotic cells by peritoneal macrophages. The in vivo functions of Tim-4 in phagocytosis and immune responses, however, are still unclear. In this study, we show that Tim-4 quickly forms punctate caps on contact with apoptotic cells, in contrast to its normal diffused expression on the surface of phagocytes. Despite its expression in marginal zone and tingible body macrophages, Tim-4 deficiency only minimally affects outcomes of several acute immune challenges, including the trapping of apoptotic cells in the marginal zone, the clearance apoptotic cells by tingible body macrophages, and the formation of germinal centers and elicitation of antibody responses against sheep red blood cells (SRBCs). In addition, Tim-4(-/-) resident peritoneal macrophages (rPMs) phagocytose necrotic cells and other opsonized targets normally. However, their ability to bind and engulf apoptotic cells is significantly compromised both in vitro and in vivo. Most importantly, Tim-4 deficiency results in increased cellularity in the peritoneum. Resting rPMs produce higher TNF-alpha in culture. Their response to LPS, on the contrary, is dampened. Our data support an indispensible role of Tim-4 in maintaining the homeostasis of rPMs.
Conflict of interest statement
Conflict of interest statement: All authors are employees of Genentech, Inc.
Figures

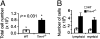
References
-
- McIntire JJ, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. - PubMed
-
- Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nature Review Immunology. 2008;8:577–580. - PubMed
-
- Meyers JH, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. - PubMed
-
- Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

