Alternate protein kinase A activity identifies a unique population of stromal cells in adult bone
- PMID: 20421483
- PMCID: PMC2889322
- DOI: 10.1073/pnas.1003680107
Alternate protein kinase A activity identifies a unique population of stromal cells in adult bone
Abstract
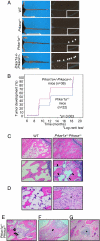
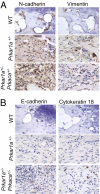
A population of stromal cells that retains osteogenic capacity in adult bone (adult bone stromal cells or aBSCs) exists and is under intense investigation. Mice heterozygous for a null allele of prkar1a (Prkar1a(+/-)), the primary receptor for cyclic adenosine monophosphate (cAMP) and regulator of protein kinase A (PKA) activity, developed bone lesions that were derived from cAMP-responsive osteogenic cells and resembled fibrous dysplasia (FD). Prkar1a(+/-) mice were crossed with mice that were heterozygous for catalytic subunit Calpha (Prkaca(+/-)), the main PKA activity-mediating molecule, to generate a mouse model with double heterozygosity for prkar1a and prkaca (Prkar1a(+/-)Prkaca(+/-)). Unexpectedly, Prkar1a(+/-)Prkaca(+/-) mice developed a greater number of osseous lesions starting at 3 months of age that varied from the rare chondromas in the long bones and the ubiquitous osteochondrodysplasia of vertebral bodies to the occasional sarcoma in older animals. Cells from these lesions originated from an area proximal to the growth plate, expressed osteogenic cell markers, and showed higher PKA activity that was mostly type II (PKA-II) mediated by an alternate pattern of catalytic subunit expression. Gene expression profiling confirmed a preosteoblastic nature for these cells but also showed a signature that was indicative of mesenchymal-to-epithelial transition and increased Wnt signaling. These studies show that a specific subpopulation of aBSCs can be stimulated in adult bone by alternate PKA and catalytic subunit activity; abnormal proliferation of these cells leads to skeletal lesions that have similarities to human FD and bone tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bossis I, Stratakis CA. Minireview: PRKAR1A: normal and abnormal functions. Endocrinology. 2004;145:5452–5458. - PubMed
-
- Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression,regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–D693. - PubMed
-
- Gamm DM, Baude EJ, Uhler MD. The major catalytic subunit isoforms of cAMP-dependent protein kinase have distinct biochemical properties in vitro and in vivo. J Biol Chem. 1996;271:15736–15742. - PubMed
-
- McKnight GS, et al. Analysis of the cAMP-dependent protein kinase system using molecular genetic approaches. Recent Prog Horm Res. 1988;44:307–335. - PubMed
-
- Kirschner LS, et al. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–4514. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

