Requirement of CCL17 for CCR7- and CXCR4-dependent migration of cutaneous dendritic cells
- PMID: 20421491
- PMCID: PMC2889308
- DOI: 10.1073/pnas.0906126107
Requirement of CCL17 for CCR7- and CXCR4-dependent migration of cutaneous dendritic cells
Abstract
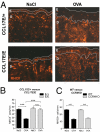
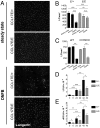
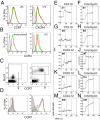
Chemokines are known to regulate the steady-state and inflammatory migration of cutaneous dendritic cells (DCs). The beta-chemokine CCL17, a ligand of CCR4, is inducibly expressed in a subset of DCs and is strongly up-regulated in atopic diseases. Using an atopic dermatitis model, we show that CCL17-deficient mice develop acanthosis as WT mice, whereas dermal inflammation, T helper 2-type cytokine production, and the allergen-specific humoral immune response are significantly decreased. Notably, CCL17-deficient mice retained Langerhans cells (LCs) in the lesional skin after chronic allergen exposure, whereas most LCs emigrated from the epidermis of allergen-treated WT controls into draining lymph nodes (LNs). Moreover, CCL17-deficient LCs showed impaired emigration from the skin after exposure to a contact sensitizer. In contrast, the absence of CCR4 had no effect on cutaneous DC migration and development of atopic dermatitis symptoms. As an explanation for the major migratory defect of CCL17-deficient DCs in vivo, we demonstrate impaired mobility of CCL17-deficient DCs to CCL19/21 in 3D in vitro migration assays and a blockade of intracellular calcium release in response to CCR7 ligands. In addition, responsiveness of CCL17-deficient DCs to CXCL12 was impaired as well. We demonstrate that the inducible chemokine CCL17 sensitizes DCs for CCR7- and CXCR4-dependent migration to LN-associated homeostatic chemokines under inflammatory conditions and thus plays an important role in cutaneous DC migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
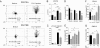
Similar articles
-
Cutting edge: identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity.J Immunol. 2013 Nov 15;191(10):4903-7. doi: 10.4049/jimmunol.1302175. Epub 2013 Oct 11. J Immunol. 2013. PMID: 24123684 Free PMC article.
-
CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells.Am J Pathol. 2007 Oct;171(4):1249-57. doi: 10.2353/ajpath.2007.070225. Epub 2007 Sep 6. Am J Pathol. 2007. PMID: 17823289 Free PMC article.
-
The glucocorticoid-induced TNF receptor-related protein (GITR)-GITR ligand pathway acts as a mediator of cutaneous dendritic cell migration and promotes T cell-mediated acquired immunity.J Immunol. 2009 Mar 1;182(5):2708-16. doi: 10.4049/jimmunol.0803704. J Immunol. 2009. PMID: 19234165
-
A two-step model for Langerhans cell migration to skin-draining LN.Eur J Immunol. 2008 Nov;38(11):2975-80. doi: 10.1002/eji.200838919. Eur J Immunol. 2008. PMID: 18991275 Free PMC article. Review.
-
Epidermal Langerhans cell migration and sensitisation to chemical allergens.APMIS. 2003 Jul-Aug;111(7-8):797-804. doi: 10.1034/j.1600-0463.2003.11107811.x. APMIS. 2003. PMID: 12974781 Review.
Cited by
-
Conditions Mimicking the Cancer Microenvironment Modulate the Functional Outcome of Human Chorionic Villus Mesenchymal Stem/Stromal Cells in vitro.Front Cell Dev Biol. 2021 Jun 21;9:650125. doi: 10.3389/fcell.2021.650125. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34235143 Free PMC article.
-
Glyteer, Soybean Tar, Impairs IL-4/Stat6 Signaling in Murine Bone Marrow-Derived Dendritic Cells: The Basis of Its Therapeutic Effect on Atopic Dermatitis.Int J Mol Sci. 2018 Apr 12;19(4):1169. doi: 10.3390/ijms19041169. Int J Mol Sci. 2018. PMID: 29649105 Free PMC article.
-
The Role of Skin Barrier in the Pathogenesis of Food Allergy.Children (Basel). 2015 Sep 2;2(3):382-402. doi: 10.3390/children2030382. Children (Basel). 2015. PMID: 27417371 Free PMC article. Review.
-
Cross-talk between Colon Cells and Macrophages Increases ST6GALNAC1 and MUC1-sTn Expression in Ulcerative Colitis and Colitis-Associated Colon Cancer.Cancer Immunol Res. 2020 Feb;8(2):167-178. doi: 10.1158/2326-6066.CIR-19-0514. Epub 2019 Dec 12. Cancer Immunol Res. 2020. PMID: 31831633 Free PMC article.
-
TNF and granulocyte macrophage-colony stimulating factor interdependence mediates inflammation via CCL17.JCI Insight. 2018 Mar 22;3(6):e99249. doi: 10.1172/jci.insight.99249. JCI Insight. 2018. PMID: 29563337 Free PMC article.
References
-
- Allen SJ, Crown SE, Handel TM. Chemokine: Receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. - PubMed
-
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. - PubMed
-
- Koch S, Kohl K, Klein E, von Bubnoff D, Bieber T. Skin homing of Langerhans cell precursors: Adhesion, chemotaxis, and migration. J Allergy Clin Immunol. 2006;117:163–168. - PubMed
-
- Randolph GJ, Ochando J, Partida-Sánchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. - PubMed
-
- Ohl L, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

