Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts
- PMID: 20421520
- PMCID: PMC2879145
- DOI: 10.1161/CIRCULATIONAHA.109.909093
Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts
Abstract
Background: C-kit is a receptor tyrosine kinase family member expressed in hematopoietic stem cells. C-kit is also transiently expressed in cardiomyocyte precursors during development and in a rare cell population in the normal adult heart. In the present study, the cardiomyogenic potential of c-kit(+) cells isolated from normal neonatal, normal adult, and infarcted adult mouse hearts was evaluated.
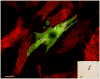
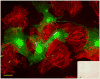
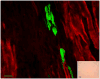
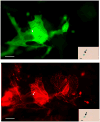
Methods and results: Magnetic activated cell sorting was used to prepare c-kit(+) cells from the hearts of ACT-EGFP/MHC-nLAC double transgenic mice. These animals exhibit widespread enhanced green fluorescent protein (EGFP) expression and cardiomyocyte-restricted nuclear beta-galactosidase activity, thus permitting simultaneous tracking of cell survival and differentiation. A subset of the c-kit(+) cells from double transgenic neonatal hearts acquired a cardiomyogenic phenotype when cocultured with fetal cardiomyocytes (2.4% of all EGFP(+) cells screened) but rarely when cultured alone or when cocultured with mouse fibroblasts (0.03% and 0.05% of the EGFP(+) cells screened, respectively). In contrast, c-kit(+) cells from normal adult double transgenic hearts failed to undergo cardiomyogenic differentiation when cocultured with nontransgenic fetal cardiomyocytes (>18 000 EGFP(+) cells screened) or when transplanted into normal or infarcted adult mouse hearts (14 EGFP(+) grafts examined). A single c-kit(+) cell from an infarcted double transgenic adult heart was observed to acquire a cardiomyogenic phenotype in coculture (>37 000 EGFP(+) cells screened).
Conclusions: These data suggest that the ability of cardiac-resident c-kit(+) cells to acquire a cardiomyogenic phenotype is subject to temporal limitations or, alternatively, that the cardiomyogenic population is lost. Elucidation of the underlying molecular basis may permit robust cardiomyogenic induction in adult-derived cardiac c-kit(+) cells.
Conflict of interest statement
None.
Figures




Comment in
-
Heart to heart: The elusive mechanism of cell therapy.Circulation. 2010 May 11;121(18):1981-4. doi: 10.1161/CIRCULATIONAHA.110.952580. Epub 2010 Apr 26. Circulation. 2010. PMID: 20421516 No abstract available.
Similar articles
-
c-kit expression identifies cardiovascular precursors in the neonatal heart.Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1808-13. doi: 10.1073/pnas.0808920106. Epub 2009 Feb 4. Proc Natl Acad Sci U S A. 2009. PMID: 19193854 Free PMC article.
-
Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction.Blood. 2004 Dec 1;104(12):3581-7. doi: 10.1182/blood-2004-04-1488. Epub 2004 Aug 5. Blood. 2004. PMID: 15297308
-
Adult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardium.Mol Ther. 2008 Jun;16(6):1129-37. doi: 10.1038/mt.2008.64. Epub 2008 Apr 22. Mol Ther. 2008. PMID: 18431364 Free PMC article.
-
Mesenchymal, but not hematopoietic, stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction in mice.Kidney Int. 2005 Nov;68(5):1940-3. doi: 10.1111/j.1523-1755.2005.00624.x. Kidney Int. 2005. PMID: 16221170 Review.
-
"String theory" of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results.Circ Res. 2015 Mar 27;116(7):1216-30. doi: 10.1161/CIRCRESAHA.116.305557. Circ Res. 2015. PMID: 25814683 Free PMC article. Review.
Cited by
-
Signaling and transcriptional networks in heart development and regeneration.Cold Spring Harb Perspect Biol. 2013 Mar 1;5(3):a008292. doi: 10.1101/cshperspect.a008292. Cold Spring Harb Perspect Biol. 2013. PMID: 23457256 Free PMC article. Review.
-
Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration.J Mol Cell Cardiol. 2011 Oct;51(4):619-25. doi: 10.1016/j.yjmcc.2011.05.015. Epub 2011 May 30. J Mol Cell Cardiol. 2011. PMID: 21645519 Free PMC article. Review.
-
Calcium-dependent potassium channels control proliferation of cardiac progenitor cells and bone marrow-derived mesenchymal stem cells.J Physiol. 2018 Jun;596(12):2359-2379. doi: 10.1113/JP275388. Epub 2018 May 5. J Physiol. 2018. PMID: 29574723 Free PMC article.
-
c-kitpos GATA-4 high rat cardiac stem cells foster adult cardiomyocyte survival through IGF-1 paracrine signalling.PLoS One. 2010 Dec 13;5(12):e14297. doi: 10.1371/journal.pone.0014297. PLoS One. 2010. PMID: 21179204 Free PMC article.
-
Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes.Cell Res. 2016 Jan;26(1):119-30. doi: 10.1038/cr.2015.143. Epub 2015 Dec 4. Cell Res. 2016. PMID: 26634606 Free PMC article.
References
-
- Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annual Review Of Physiology. 2006;68:29–49. - PubMed
-
- Edling CE, Hallberg B. c-Kit--a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol. 2007;39:1995–1998. - PubMed
-
- Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. - PubMed
-
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental Origin of a Bipotential Myocardial and Smooth Muscle Cell Precursor in the Mammalian Heart. Cell. 2006;127:1137–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

