Effect of translesion DNA polymerases, endonucleases and RpoS on mutation rates in Salmonella typhimurium
- PMID: 20421601
- PMCID: PMC2907201
- DOI: 10.1534/genetics.110.116376
Effect of translesion DNA polymerases, endonucleases and RpoS on mutation rates in Salmonella typhimurium
Abstract
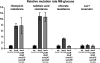
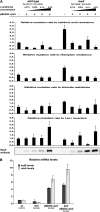
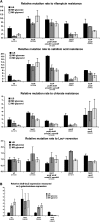
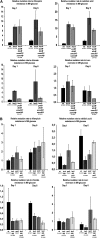
It has been suggested that bacteria have evolved mechanisms to increase their mutation rate in response to various stresses and that the translesion DNA polymerase Pol IV under control of the LexA regulon and the alternative sigma factor RpoS are involved in regulating this mutagenesis. Here we examined in Salmonella enterica serovar Typhimurium LT2 the rates for four different types of mutations (rifampicin, nalidixic acid, and chlorate resistance and Lac(+) reversion) during various growth conditions and with different levels of four translesion DNA polymerases (Pol II, Pol IV, Pol V, and SamAB) and RpoS. Constitutive derepression of the LexA regulon by a lexA(def) mutation had no effect on Lac(+) reversion rates but increased the other three mutation rates up to 11-fold, and the contribution of the translesion DNA polymerases to this mutagenesis varied with the type of mutation examined. The increase in mutation rates in the lexA(def) mutant required the presence of the LexA-controlled UvrB protein and endonucleases UvrC and Cho. With regard to the potential involvement of RpoS in mutagenesis, neither an increase in RpoS levels conferred by artificial overexpression from a plasmid nor long-term stationary phase incubation or slow growth caused an increase in any of the four mutation rates measured, alone or in combination with overexpression of the translesion DNA polymerases. In conclusion, mutation rates are remarkably robust and no combination of growth conditions, induction of translesion DNA polymerases by inactivation of LexA, or increased RpoS expression could confer an increase in mutation rates higher than the moderate increase caused by derepression of the LexA regulon alone.
Figures
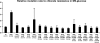
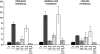
Similar articles
-
Translesion DNA polymerases are required for spontaneous deletion formation in Salmonella typhimurium.Proc Natl Acad Sci U S A. 2009 Jun 23;106(25):10248-53. doi: 10.1073/pnas.0904389106. Epub 2009 Jun 12. Proc Natl Acad Sci U S A. 2009. PMID: 19525399 Free PMC article.
-
Rapid evolution of acetic acid-detoxifying Escherichia coli under phosphate starvation conditions requires activation of the cryptic PhnE permease and induction of translesion synthesis DNA polymerases.FEMS Microbiol Lett. 2017 Feb 1;364(4). doi: 10.1093/femsle/fnx031. FEMS Microbiol Lett. 2017. PMID: 28199639
-
Identification of RpoS (sigma(S))-regulated genes in Salmonella enterica serovar typhimurium.J Bacteriol. 2000 Oct;182(20):5749-56. doi: 10.1128/JB.182.20.5749-5756.2000. J Bacteriol. 2000. PMID: 11004173 Free PMC article.
-
Environmental tuning of mutation rates.Environ Microbiol. 2006 Feb;8(2):193-9. doi: 10.1111/j.1462-2920.2005.00968.x. Environ Microbiol. 2006. PMID: 16423008 Review.
-
Mutations for Worse or Better: Low-Fidelity DNA Synthesis by SOS DNA Polymerase V Is a Tightly Regulated Double-Edged Sword.Biochemistry. 2016 Apr 26;55(16):2309-18. doi: 10.1021/acs.biochem.6b00117. Epub 2016 Apr 12. Biochemistry. 2016. PMID: 27043933 Free PMC article. Review.
Cited by
-
Roles of the Y-family DNA polymerase Dbh in accurate replication of the Sulfolobus genome at high temperature.DNA Repair (Amst). 2012 Apr 1;11(4):391-400. doi: 10.1016/j.dnarep.2012.01.005. Epub 2012 Feb 4. DNA Repair (Amst). 2012. PMID: 22305938 Free PMC article.
-
Impact of a stress-inducible switch to mutagenic repair of DNA breaks on mutation in Escherichia coli.Proc Natl Acad Sci U S A. 2011 Aug 16;108(33):13659-64. doi: 10.1073/pnas.1104681108. Epub 2011 Aug 1. Proc Natl Acad Sci U S A. 2011. PMID: 21808005 Free PMC article.
-
Thermal Resistance and Gene Expression of both Desiccation-Adapted and Rehydrated Salmonella enterica Serovar Typhimurium Cells in Aged Broiler Litter.Appl Environ Microbiol. 2017 May 31;83(12):e00367-17. doi: 10.1128/AEM.00367-17. Print 2017 Jun 15. Appl Environ Microbiol. 2017. PMID: 28389541 Free PMC article.
-
Regulation of nucleotide excision repair activity by transcriptional and post-transcriptional control of the XPA protein.Nucleic Acids Res. 2011 Apr;39(8):3176-87. doi: 10.1093/nar/gkq1318. Epub 2010 Dec 30. Nucleic Acids Res. 2011. PMID: 21193487 Free PMC article.
-
Translesion DNA Synthesis.EcoSal Plus. 2012 Nov;5(1):10.1128/ecosalplus.7.2.2. doi: 10.1128/ecosalplus.7.2.2. EcoSal Plus. 2012. PMID: 26442823 Free PMC article.
References
-
- Andersson, D. I., E. S. Slechta and J. R. Roth, 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282(5391): 1133–1135. - PubMed
-
- Bhamre, S., B. B. Gadea, C. A. Koyama, S. J. White and R. G. Fowler, 2001. An aerobic recA-, umuC-dependent pathway of spontaneous base-pair substitution mutagenesis in Escherichia coli. Mutat. Res. 473(2): 229–247. - PubMed
-
- Bjedov, I., O. Tenaillon, B. Gérard, V. Souza, E. Denamur et al., 2003. Stress-induced mutagenesis in bacteria. Science 300(5624): 1404–1409. - PubMed
-
- Branum, M. E., J. T. Reardon and A. Sancar, 2001. DNA repair excision nuclease attacks undamaged DNA. A potential source of spontaneous mutations. J. Biol. Chem. 276(27): 25421–25426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

