Inhibition of VEGF- and NO-dependent angiogenesis does not impair liver regeneration
- PMID: 20421635
- PMCID: PMC2867515
- DOI: 10.1152/ajpregu.00836.2009
Inhibition of VEGF- and NO-dependent angiogenesis does not impair liver regeneration
Abstract
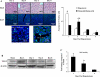
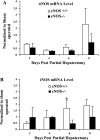
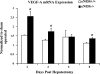
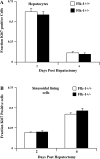
Angiogenesis occurs through a convergence of diverse signaling mechanisms with prominent pathways that include autocrine effects of endothelial nitric oxide (NO) synthase (eNOS)-derived NO and vascular endothelial growth factor (VEGF). However, the redundant and distinct roles of NO and VEGF in angiogenesis remain incompletely defined. Here, we use the partial hepatectomy model in mice genetically deficient in eNOS to ascertain the influence of eNOS-derived NO on the angiogenesis that accompanies liver regeneration. While sinusoidal endothelial cell (SEC) eNOS promotes angiogenesis in vitro, surprisingly the absence of eNOS did not influence the angiogenesis that occurs after partial hepatectomy in vivo. While this observation could not be attributed to induction of alternate NOS isoforms, it was associated with induction of VEGF signaling as evidenced by enhanced levels of VEGF ligand in regenerating livers from mice genetically deficient in eNOS. However, surprisingly, mice that were genetically heterozygous for deficiency in the VEGF receptor, fetal liver kinase-1, also maintained unimpaired capacity for liver regeneration. In summary, inhibition of VEGF- and NO-dependent angiogenesis does not impair liver regeneration, indicating signaling redundancies that allow liver regeneration to continue in the absence of this canonical vascular pathway.
Figures


Similar articles
-
Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice.Hepatology. 2011 Nov;54(5):1777-89. doi: 10.1002/hep.24560. Epub 2011 Oct 11. Hepatology. 2011. PMID: 21748771 Free PMC article.
-
Roles of vascular endothelial growth factor and endothelial nitric oxide synthase during revascularization and regeneration after partial hepatectomy in a rat model.Surg Today. 2011 Dec;41(12):1622-9. doi: 10.1007/s00595-010-4484-9. Epub 2011 Oct 4. Surg Today. 2011. PMID: 21969195
-
[Effect of nitric oxide derived from endothelial nitric oxide synthase on tumor angiogenesis].Chin J Cancer. 2010 Jan;29(1):32-7. doi: 10.5732/cjc.009.10246. Chin J Cancer. 2010. PMID: 20038308 Chinese.
-
The vascular endothelial growth factor signaling pathway regulates liver sinusoidal endothelial cells during liver regeneration after partial hepatectomy.Expert Rev Gastroenterol Hepatol. 2021 Feb;15(2):139-147. doi: 10.1080/17474124.2020.1815532. Epub 2020 Oct 14. Expert Rev Gastroenterol Hepatol. 2021. PMID: 32902336 Review.
-
Vascular endothelial growth factor protein and gene delivery by novel nanomaterials for promoting liver regeneration after partial hepatectomy.World J Gastroenterol. 2023 Jun 28;29(24):3748-3757. doi: 10.3748/wjg.v29.i24.3748. World J Gastroenterol. 2023. PMID: 37426320 Free PMC article. Review.
Cited by
-
The effect of sorafenib on liver regeneration and angiogenesis after partial hepatectomy in rats.Hippokratia. 2015 Jul-Sep;19(3):249-55. Hippokratia. 2015. PMID: 27418785 Free PMC article.
-
Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice.Hepatology. 2011 Nov;54(5):1777-89. doi: 10.1002/hep.24560. Epub 2011 Oct 11. Hepatology. 2011. PMID: 21748771 Free PMC article.
-
Angiogenic effects of apigenin on endothelial cells after hypoxia-reoxygenation via the caveolin-1 pathway.Int J Mol Med. 2017 Dec;40(6):1639-1648. doi: 10.3892/ijmm.2017.3159. Epub 2017 Sep 28. Int J Mol Med. 2017. PMID: 29039442 Free PMC article.
-
Adenosine A2B receptor stimulates angiogenesis by inducing VEGF and eNOS in human microvascular endothelial cells.Exp Biol Med (Maywood). 2015 Nov;240(11):1472-9. doi: 10.1177/1535370215584939. Epub 2015 May 12. Exp Biol Med (Maywood). 2015. PMID: 25966978 Free PMC article.
-
Ticagrelor Induces Angiogenesis in Progenitor and Mature Endothelial Cells In Vitro: Investigation of the Possible Role of Adenosine.Int J Mol Sci. 2024 Dec 12;25(24):13343. doi: 10.3390/ijms252413343. Int J Mol Sci. 2024. PMID: 39769108 Free PMC article.
References
-
- Assy N, Spira G, Paizi M, Shenkar L, Kraizer Y, Cohen T, Neufeld G, Dabbah B, Enat R, Baruch Y. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol 30: 911–915, 1999 - PubMed
-
- Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grunewald P, Trippler M, Biglarnia A, Kamler M, Niehues EM, Frilling A, Broelsch CE, Schlaak JF. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res 138: 291–299, 2007 - PubMed
-
- Bosch J. Vascular deterioration in cirrhosis: the big picture. J Clin Gastroenterol 41, Suppl 3: S247–S253, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

