CD28 facilitates the generation of Foxp3(-) cytokine responsive regulatory T cell precursors
- PMID: 20421644
- PMCID: PMC2874117
- DOI: 10.4049/jimmunol.1000019
CD28 facilitates the generation of Foxp3(-) cytokine responsive regulatory T cell precursors
Abstract
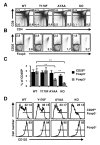
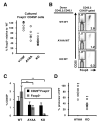
The T cell costimulatory molecule CD28 plays an important role in the thymic generation of Foxp3(+) regulatory T cells (Tregs) essential for the maintenance of self-tolerance. In this study, we show that a cell-intrinsic signal from CD28 is involved in the generation of cytokine-responsive Foxp3(-) precursors using studies of mixed bone marrow chimeras as well as TCR-specific generation of Foxp3(+) cells using intrathymic transfer of TCR-transgenic thymocytes expressing a natural Treg TCR. Contrary to a previous report, the analysis of CD28 mutant knockin mice revealed that this cell-intrinsic signal is only partially dependent on the Lck-binding PYAP motif. Surprisingly, even though the absence of CD28 resulted in a 6-fold decrease in thymic Tregs, the TCR repertoires of CD28-deficient and sufficient cells were largely overlapping. Thus, these data suggest that CD28 does not operate by markedly enlarging the repertoire of TCRs available for Treg development, but rather by improving the efficiency of Treg development of thymocytes expressing natural Treg TCRs.
Figures



References
-
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nature Immunology. 2005;6:331–337. - PubMed
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
-
- Jordan MS, Boesteanu A, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunology. 2001;2:301–306. - PubMed
-
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature Immunology. 2002;3:756–763. - PubMed
-
- Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nature Immunology. 2001;2:816–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

