All-trans retinoic acid (ATRA) downregulates MMP-9 by modulating its regulatory molecules
- PMID: 20421725
- PMCID: PMC2958618
- DOI: 10.4161/cam.4.3.11682
All-trans retinoic acid (ATRA) downregulates MMP-9 by modulating its regulatory molecules
Abstract
The vitamin A derivative all-trans retinoic acid (ATRA) is considered as a potent chemotherapeutic drug for its capability of regulating cell growth and differentiation. We aimed to study the effect of ATRA on MMP-9 in MDA-MB-231, human breast cancer cells and the probable molecular mechanisms through which ATRA exerts its effect.
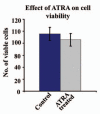
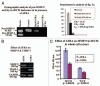
Results: Our experimental findings demonstrate that ATRA enters into the nucleus and regulates various signaling pathways viz. Integrin, FAK, ERK, PI-3K, NF-κB and also EGFR and down regulates pro-MMP-9 activity as well as its expression. As a result MDA-MB-231 cell migration on fibronectin medium gets retarded in presence of ATRA. ATRA up regulates TIMP-1 expression. Our study may help to understand the role of ATRA as a regulator of MMP-9 and the possible signaling pathways which are involved in this ATRA mediated down regulation of MMP-9.
Figures

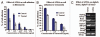
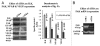



Similar articles
-
Culture of human breast cancer cell line (MDA-MB-231) on fibronectin-coated surface induces pro-matrix metalloproteinase-9 expression and activity.Tumour Biol. 2011 Feb;32(1):129-38. doi: 10.1007/s13277-010-0106-9. Epub 2010 Sep 7. Tumour Biol. 2011. PMID: 20821288
-
Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B (MMP-9) by involvement of FAK/ERK/NFkappaB and AP-1 in the human breast cancer cell line MDA-MB-231.Anticancer Drugs. 2010 Jul;21(6):632-44. doi: 10.1097/cad.0b013e32833a4385. Anticancer Drugs. 2010. PMID: 20527725
-
Extracellular matrix protein fibronectin induces matrix metalloproteinases in human prostate adenocarcinoma cells PC-3.Cell Commun Adhes. 2013 Oct;20(5):105-14. doi: 10.3109/15419061.2013.833193. Epub 2013 Sep 19. Cell Commun Adhes. 2013. PMID: 24047237
-
The potential mechanism for the different expressions of gelatinases induced by all-trans retinoic acid in different cells.J Recept Signal Transduct Res. 2012 Jun;32(3):129-33. doi: 10.3109/10799893.2012.672992. Epub 2012 Apr 5. J Recept Signal Transduct Res. 2012. PMID: 22475041 Review.
-
Potential Therapeutic Effect of All-Trans Retinoic Acid on Atherosclerosis.Biomolecules. 2022 Jun 22;12(7):869. doi: 10.3390/biom12070869. Biomolecules. 2022. PMID: 35883425 Free PMC article. Review.
Cited by
-
ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion.Nat Commun. 2016 Sep 7;7:12630. doi: 10.1038/ncomms12630. Nat Commun. 2016. PMID: 27600527 Free PMC article.
-
Altered vitamin A metabolism in human liver slices corresponds to fibrogenesis.Clin Transl Sci. 2021 May;14(3):976-989. doi: 10.1111/cts.12962. Epub 2021 Feb 2. Clin Transl Sci. 2021. PMID: 33382909 Free PMC article.
-
Dysregulated retinoic acid signaling in airway smooth muscle cells in asthma.FASEB J. 2021 Dec;35(12):e22016. doi: 10.1096/fj.202100835R. FASEB J. 2021. PMID: 34784434 Free PMC article.
-
Recent progress in natural dietary non-phenolic bioactives on cancers metastasis.J Food Drug Anal. 2018 Jul;26(3):940-964. doi: 10.1016/j.jfda.2018.05.003. Epub 2018 Jun 1. J Food Drug Anal. 2018. PMID: 29976413 Free PMC article. Review.
-
All-trans-retinoic acid reduces BACE1 expression under inflammatory conditions via modulation of nuclear factor κB (NFκB) signaling.J Biol Chem. 2015 Sep 11;290(37):22532-42. doi: 10.1074/jbc.M115.662908. Epub 2015 Aug 3. J Biol Chem. 2015. PMID: 26240147 Free PMC article.
References
-
- Silveira ER, Naves MM, Vannucchi H, Jordao Junior AA, Dagli ML, Moreno FS. Vitamin A and all-trans and 9-cis retinoic acids inhibit cell proliferation during the progression phase of hepatocarcinogenesis in Wistar rats. Nutr Cancer. 2001;39:244–251. - PubMed
-
- Orlandi M, Mantovani B, Ammar K, Avitabile E, Dal Monte P, Bartolini G. Retinoids and cancer: antitumoral effects of ATRA, 9-cis RA and the new retinoid IIF on the HL-60 leukemic cell line. Med Princ Pract. 2003;12:164–169. - PubMed
-
- Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Ann Rev Nutr. 2004;24:201–221. - PubMed
-
- Liu H, Zang C, Fenner MH, Possinger K, Elstner E. PPARgamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat. 2003;79:63–74. - PubMed
-
- Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g., acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
