Regulation of human Th9 differentiation by type I interferons and IL-21
- PMID: 20421880
- PMCID: PMC3090036
- DOI: 10.1038/icb.2010.53
Regulation of human Th9 differentiation by type I interferons and IL-21
Abstract
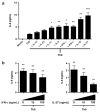
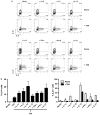
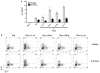
Interleukin (IL)-9-producing CD4(+) T cells are a novel subset of T helper (Th) cells that develops independently of the Th1, Th2, Th17 and regulatory T-cell lineages. Similar to the murine model, transforming growth factor (TGF)-beta and IL-4 directed human naive CD4(+) T cells to produce IL-9. Whereas IL-4 suppressed TGF-beta-induced Foxp3 expression, TGF-beta failed to inhibit IL-4-mediated upregulation of the Th2 transcription factor GATA-3. Addition of IL-1 beta, IL-6, IL-10, interferon (IFN)-alpha, IFN-beta or IL-21 to Th9-polarizing conditions augmented Th9 differentiation, while the Th1-associated cytokines IFN-gamma and IL-27 partially suppressed IL-9 production. Given that T cells are a primary source of IL-21, IL-21 expression was analyzed under Th9-polarizing conditions in the context of inflammatory cytokines. Surprisingly, type I IFNs induced elevated levels of IL-21, and blockade of IL-21 abrogated their ability to enhance Th9 differentiation. Taken together, these data indicate a complex cytokine network in the regulation of human IL-9-producing CD4(+) T cells.
Conflict of interest statement
We state the following conflict of interest disclosures over the past 3 years: PJU has served as a consultant to Centocor (Horsham, PA, USA), Biogen Idec (Cambridge, MA, USA), Genentech, Inc. (South San Francisco, CA, USA), Astra Zeneca (London, UK), CoMentis (South San Francisco, CA, USA), Gilead Sciences (Foster City, CA, USA), Regimmune (Mountain View, CA, USA) and UCB (Belgium), and is a co-founder and consultant at Bayhill Therapeutics (San Mateo, CA, USA). EE is on the scientific advisory board of Globeimmune, Inc.
Figures
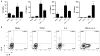

Comment in
-
Human Th9 cells: inflammatory cytokines modulate IL-9 production through the induction of IL-21.Immunol Cell Biol. 2010 Aug;88(6):621-3. doi: 10.1038/icb.2010.73. Epub 2010 Jun 8. Immunol Cell Biol. 2010. PMID: 20531361 No abstract available.
Similar articles
-
Flow Cytometric Assessment of STAT Molecules in Th9 Cells.Methods Mol Biol. 2017;1585:127-140. doi: 10.1007/978-1-4939-6877-0_10. Methods Mol Biol. 2017. PMID: 28477192
-
TGF-beta induces IL-9 production from human Th17 cells.J Immunol. 2010 Jul 1;185(1):46-54. doi: 10.4049/jimmunol.1000356. Epub 2010 May 24. J Immunol. 2010. PMID: 20498357 Free PMC article.
-
A Method to In Vitro Differentiate Th9 Cells from Mouse Naïve CD4+ T Cells.Methods Mol Biol. 2017;1585:51-57. doi: 10.1007/978-1-4939-6877-0_4. Methods Mol Biol. 2017. PMID: 28477186 Free PMC article.
-
Th9 cells, new players in adaptive immunity.Trends Immunol. 2014 Feb;35(2):61-8. doi: 10.1016/j.it.2013.10.004. Epub 2013 Nov 8. Trends Immunol. 2014. PMID: 24215739 Review.
-
Transcriptional Control of Th9 Cells: Role of Foxo1 in Interleukin-9 Induction.Front Immunol. 2018 May 9;9:995. doi: 10.3389/fimmu.2018.00995. eCollection 2018. Front Immunol. 2018. PMID: 29867972 Free PMC article. Review.
Cited by
-
A deleterious role for Th9/IL-9 in hepatic fibrogenesis.Sci Rep. 2016 Jan 5;6:18694. doi: 10.1038/srep18694. Sci Rep. 2016. PMID: 26728971 Free PMC article.
-
T cell subsets: an integral component in pathogenesis of rheumatic heart disease.Immunol Res. 2018 Feb;66(1):18-30. doi: 10.1007/s12026-017-8978-z. Immunol Res. 2018. PMID: 29170852 Review.
-
IL-4-, TGF-β-, and IL-1-dependent expansion of parasite antigen-specific Th9 cells is associated with clinical pathology in human lymphatic filariasis.J Immunol. 2013 Sep 1;191(5):2466-73. doi: 10.4049/jimmunol.1300911. Epub 2013 Aug 2. J Immunol. 2013. PMID: 23913964 Free PMC article.
-
A gender-related action of IFNbeta-therapy was found in multiple sclerosis.J Transl Med. 2012 Nov 14;10:223. doi: 10.1186/1479-5876-10-223. J Transl Med. 2012. PMID: 23148845 Free PMC article.
-
IL-33 induces IL-9 production in human CD4+ T cells and basophils.PLoS One. 2011;6(7):e21695. doi: 10.1371/journal.pone.0021695. Epub 2011 Jul 6. PLoS One. 2011. PMID: 21765905 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

