Mosquito transcriptome profiles and filarial worm susceptibility in Armigeres subalbatus
- PMID: 20421927
- PMCID: PMC2857672
- DOI: 10.1371/journal.pntd.0000666
Mosquito transcriptome profiles and filarial worm susceptibility in Armigeres subalbatus
Abstract
Background: Armigeres subalbatus is a natural vector of the filarial worm Brugia pahangi, but it kills Brugia malayi microfilariae by melanotic encapsulation. Because B. malayi and B. pahangi are morphologically and biologically similar, comparing Ar. subalbatus-B. pahangi susceptibility and Ar. subalbatus-B. malayi refractoriness could provide significant insight into recognition mechanisms required to mount an effective anti-filarial worm immune response in the mosquito, as well as provide considerable detail into the molecular components involved in vector competence. Previously, we assessed the transcriptional response of Ar. subalbatus to B. malayi, and now we report transcriptome profiling studies of Ar. subalbatus in relation to filarial worm infection to provide information on the molecular components involved in B. pahangi susceptibility.
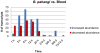
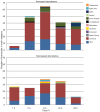
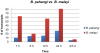
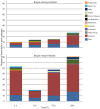
Methodology/principal findings: Utilizing microarrays, comparisons were made between mosquitoes exposed to B. pahangi, B. malayi, and uninfected bloodmeals. The time course chosen facilitated an examination of key events in the development of the parasite, beginning with the very start of filarial worm infection and spanning to well after parasites had developed to the infective stage in the mosquito. At 1, 3, 6, 12, 24 h post infection and 2-3, 5-6, 8-9, and 13-14 days post challenge there were 31, 75, 113, 76, 54, 5, 3, 13, and 2 detectable transcripts, respectively, with significant differences in transcript abundance (increase or decrease) as a result of parasite development.
Conclusions/significance: Herein, we demonstrate that filarial worm susceptibility in a laboratory strain of the natural vector Ar. subalbatus involves many factors of both known and unknown function that most likely are associated with filarial worm penetration through the midgut, invasion into thoracic muscle cells, and maintenance of homeostasis in the hemolymph environment. The data show that there are distinct and separate transcriptional patterns associated with filarial worm susceptibility as compared to refractoriness, and that an infection response in Ar. subalbatus can differ significantly from that observed in Ae. aegypti, a common laboratory model.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Molyneux DH. Control of human parasitic diseases: Context and overview. Adv Parasitol. 2006;61:1–45. - PubMed
-
- Muturi EJ, Jacob BG, Kim CH, Mbogo CM, Novak RJ. Are coinfections of malaria and filariasis of any epidemiological significance? Parasitol Res. 2008;102:175–181. - PubMed
-
- Carton Y, Nappi AJ, Poirie M. Genetics of anti-parasite resistance in invertebrates. Dev Comp Immunol. 2005;29:9–32. - PubMed
-
- Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles-transmitted filariasis. Ann Trop Med Parasitol. 2002;96(Suppl 2):S143–152. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

