Deletion of the WD40 domain of LRRK2 in Zebrafish causes Parkinsonism-like loss of neurons and locomotive defect
- PMID: 20421934
- PMCID: PMC2858694
- DOI: 10.1371/journal.pgen.1000914
Deletion of the WD40 domain of LRRK2 in Zebrafish causes Parkinsonism-like loss of neurons and locomotive defect
Abstract
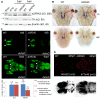
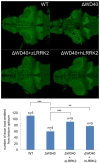
LRRK2 plays an important role in Parkinson's disease (PD), but its biological functions are largely unknown. Here, we cloned the homolog of human LRRK2, characterized its expression, and investigated its biological functions in zebrafish. The blockage of zebrafish LRRK2 (zLRRK2) protein by morpholinos caused embryonic lethality and severe developmental defects such as growth retardation and loss of neurons. In contrast, the deletion of the WD40 domain of zLRRK2 by morpholinos targeting splicing did not induce severe embryonic developmental defects; rather it caused Parkinsonism-like phenotypes, including loss of dopaminergic neurons in diencephalon and locomotion defects. These neurodegenerative and locomotion defects could be rescued by over-expressing zLRRK2 or hLRRK2 mRNA. The administration of L-dopa could also rescue the locomotion defects, but not the neurodegeneration. Taken together, our results demonstrate that zLRRK2 is an ortholog of hLRRK2 and that the deletion of WD40 domain of zLRRK2 provides a disease model for PD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Disruption of LRRK2 does not cause specific loss of dopaminergic neurons in zebrafish.PLoS One. 2011;6(6):e20630. doi: 10.1371/journal.pone.0020630. Epub 2011 Jun 16. PLoS One. 2011. PMID: 21698186 Free PMC article.
-
LRRK2 knockdown in zebrafish causes developmental defects, neuronal loss, and synuclein aggregation.J Neurosci Res. 2016 Aug;94(8):717-35. doi: 10.1002/jnr.23754. Epub 2016 Jun 5. J Neurosci Res. 2016. PMID: 27265751
-
A Drosophila model for LRRK2-linked parkinsonism.Proc Natl Acad Sci U S A. 2008 Feb 19;105(7):2693-8. doi: 10.1073/pnas.0708452105. Epub 2008 Feb 7. Proc Natl Acad Sci U S A. 2008. PMID: 18258746 Free PMC article.
-
The role of the LRRK2 gene in Parkinsonism.Mol Neurodegener. 2014 Nov 12;9:47. doi: 10.1186/1750-1326-9-47. Mol Neurodegener. 2014. PMID: 25391693 Free PMC article. Review.
-
Biochemical and molecular features of LRRK2 and its pathophysiological roles in Parkinson's disease.BMB Rep. 2010 Apr;43(4):233-44. doi: 10.5483/bmbrep.2010.43.4.233. BMB Rep. 2010. PMID: 20423607 Review.
Cited by
-
Disruption of LRRK2 does not cause specific loss of dopaminergic neurons in zebrafish.PLoS One. 2011;6(6):e20630. doi: 10.1371/journal.pone.0020630. Epub 2011 Jun 16. PLoS One. 2011. PMID: 21698186 Free PMC article.
-
Pla2g6 Deficiency in Zebrafish Leads to Dopaminergic Cell Death, Axonal Degeneration, Increased β-Synuclein Expression, and Defects in Brain Functions and Pathways.Mol Neurobiol. 2018 Aug;55(8):6734-6754. doi: 10.1007/s12035-017-0846-2. Epub 2018 Jan 17. Mol Neurobiol. 2018. PMID: 29344929
-
LIM Homeobox 4 (lhx4) regulates retinal neural differentiation and visual function in zebrafish.Sci Rep. 2021 Jan 21;11(1):1977. doi: 10.1038/s41598-021-81211-w. Sci Rep. 2021. PMID: 33479361 Free PMC article.
-
Zebrafish, Medaka and Turquoise Killifish for Understanding Human Neurodegenerative/Neurodevelopmental Disorders.Int J Mol Sci. 2022 Jan 26;23(3):1399. doi: 10.3390/ijms23031399. Int J Mol Sci. 2022. PMID: 35163337 Free PMC article. Review.
-
Studies on sensitivity of zebrafish as a model organism for Parkinson's disease: Comparison with rat model.J Pharmacol Pharmacother. 2014 Jan;5(1):39-46. doi: 10.4103/0976-500X.124422. J Pharmacol Pharmacother. 2014. PMID: 24554909 Free PMC article.
References
-
- Thomas B, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16 Spec No. 2:R183–194. - PubMed
-
- Douglas MR, Lewthwaite AJ, Nicholl DJ. Genetics of Parkinson's disease and parkinsonism. Expert Rev Neurother. 2007;7:657–666. - PubMed
-
- Schapira AH. Etiology of Parkinson's disease. Neurology. 2006;66:S10–23. - PubMed
-
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. - PubMed
-
- Ross OA. Lrrking in the background: common pathways of neurodegeneration. J Am Geriatr Soc. 2007;55:804–805. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

