Rhomboid 4 (ROM4) affects the processing of surface adhesins and facilitates host cell invasion by Toxoplasma gondii
- PMID: 20421941
- PMCID: PMC2858701
- DOI: 10.1371/journal.ppat.1000858
Rhomboid 4 (ROM4) affects the processing of surface adhesins and facilitates host cell invasion by Toxoplasma gondii
Abstract
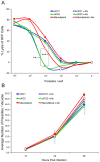
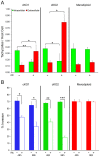
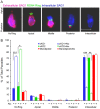
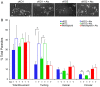
Host cell attachment by Toxoplasma gondii is dependent on polarized secretion of apical adhesins released from the micronemes. Subsequent translocation of these adhesive complexes by an actin-myosin motor powers motility and host cell invasion. Invasion and motility are also accompanied by shedding of surface adhesins by intramembrane proteolysis. Several previous studies have implicated rhomboid proteases in this step; however, their precise roles in vivo have not been elucidated. Using a conditional knockout strategy, we demonstrate that TgROM4 participates in processing of surface adhesins including MIC2, AMA1, and MIC3. Suppression of TgROM4 led to decreased release of the adhesin MIC2 into the supernatant and concomitantly increased the surface expression of this and a subset of other adhesins. Suppression of TgROM4 resulted in disruption of normal gliding, with the majority of parasites twirling on their posterior ends. Parasites lacking TgROM4 bound better to host cells, but lost the ability to apically orient and consequently most failed to generate a moving junction; hence, invasion was severely impaired. Our findings indicate that TgROM4 is involved in shedding of micronemal proteins from the cell surface. Down regulation of TgROM4 disrupts the normal apical-posterior gradient of adhesins that is important for efficient cell motility and invasion of host cells by T. gondii.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

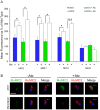
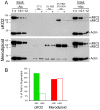
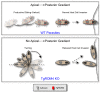
Similar articles
-
Functional analysis of rhomboid proteases during Toxoplasma invasion.mBio. 2014 Oct 21;5(5):e01795-14. doi: 10.1128/mBio.01795-14. mBio. 2014. PMID: 25336455 Free PMC article.
-
Distinct contribution of Toxoplasma gondii rhomboid proteases 4 and 5 to micronemal protein protease 1 activity during invasion.Mol Microbiol. 2015 Jul;97(2):244-62. doi: 10.1111/mmi.13021. Epub 2015 May 9. Mol Microbiol. 2015. PMID: 25846828
-
Not a Simple Tether: Binding of Toxoplasma gondii AMA1 to RON2 during Invasion Protects AMA1 from Rhomboid-Mediated Cleavage and Leads to Dephosphorylation of Its Cytosolic Tail.mBio. 2016 Sep 13;7(5):e00754-16. doi: 10.1128/mBio.00754-16. mBio. 2016. PMID: 27624124 Free PMC article.
-
Preparing for an invasion: charting the pathway of adhesion proteins to Toxoplasma micronemes.Parasitol Res. 2006 Apr;98(5):389-95. doi: 10.1007/s00436-005-0062-2. Epub 2005 Dec 30. Parasitol Res. 2006. PMID: 16385407 Review.
-
Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii.Int J Parasitol. 2001 Oct;31(12):1293-302. doi: 10.1016/s0020-7519(01)00257-0. Int J Parasitol. 2001. PMID: 11566297 Review.
Cited by
-
Marine gregarine genomes reveal the breadth of apicomplexan diversity with a partially conserved glideosome machinery.BMC Genomics. 2022 Jul 2;23(1):485. doi: 10.1186/s12864-022-08700-8. BMC Genomics. 2022. PMID: 35780080 Free PMC article.
-
Temporal gene expression during asexual development of the apicomplexan Sarcocystis neurona.mSphere. 2024 Jun 25;9(6):e0011124. doi: 10.1128/msphere.00111-24. Epub 2024 May 29. mSphere. 2024. PMID: 38809064 Free PMC article.
-
Proteomics analysis reveals that the proto-oncogene eIF-5A indirectly influences the growth, invasion and replication of Toxoplasma gondii tachyzoite.Parasit Vectors. 2021 May 26;14(1):283. doi: 10.1186/s13071-021-04791-6. Parasit Vectors. 2021. PMID: 34039408 Free PMC article.
-
Conformational change in rhomboid protease GlpG induced by inhibitor binding to its S' subsites.Biochemistry. 2012 May 8;51(18):3723-31. doi: 10.1021/bi300368b. Epub 2012 Apr 24. Biochemistry. 2012. PMID: 22515733 Free PMC article.
-
Functional Analysis of the Role of Toxoplasma gondii Nucleoside Triphosphate Hydrolases I and II in Acute Mouse Virulence and Immune Suppression.Infect Immun. 2016 Jun 23;84(7):1994-2001. doi: 10.1128/IAI.00077-16. Print 2016 Jul. Infect Immun. 2016. PMID: 27091930 Free PMC article.
References
-
- Sibley LD. Invasion strategies of intracellular parasites. Science. 2004;304:248–253. - PubMed
-
- Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. - PubMed
-
- Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

