Cdh11 acts as a tumor suppressor in a murine retinoblastoma model by facilitating tumor cell death
- PMID: 20421947
- PMCID: PMC2858707
- DOI: 10.1371/journal.pgen.1000923
Cdh11 acts as a tumor suppressor in a murine retinoblastoma model by facilitating tumor cell death
Abstract
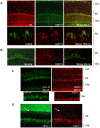
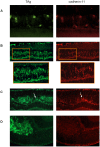
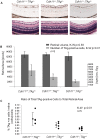
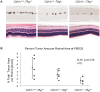
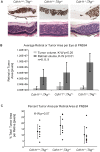
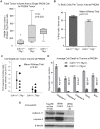
CDH11 gene copy number and expression are frequently lost in human retinoblastomas and in retinoblastomas arising in TAg-RB mice. To determine the effect of Cdh11 loss in tumorigenesis, we crossed Cdh11 null mice with TAg-RB mice. Loss of Cdh11 had no gross morphological effect on the developing retina of Cdh11 knockout mice, but led to larger retinal volumes in mice crossed with TAg-RB mice (p = 0.01). Mice null for Cdh11 presented with fewer TAg-positive cells at postnatal day 8 (PND8) (p = 0.01) and had fewer multifocal tumors at PND28 (p = 0.016), compared to mice with normal Cdh11 alleles. However, tumor growth was faster in Cdh11-null mice between PND8 and PND84 (p = 0.003). In tumors of Cdh11-null mice, cell death was decreased 5- to 10-fold (p<0.03 for all markers), while proliferation in vivo remained unaffected (p = 0.121). Activated caspase-3 was significantly decreased and beta-catenin expression increased in Cdh11 knockdown experiments in vitro. These data suggest that Cdh11 displays tumor suppressor properties in vivo and in vitro in murine retinoblastoma through promotion of cell death.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Minimal 16q genomic loss implicates cadherin-11 in retinoblastoma.Mol Cancer Res. 2004 Sep;2(9):495-503. Mol Cancer Res. 2004. PMID: 15383628
-
The TAg-RB murine retinoblastoma cell of origin has immunohistochemical features of differentiated Muller glia with progenitor properties.Invest Ophthalmol Vis Sci. 2011 Sep 29;52(10):7618-24. doi: 10.1167/iovs.11-7989. Invest Ophthalmol Vis Sci. 2011. PMID: 21862643 Free PMC article.
-
The p75 NTR neurotrophin receptor is a tumor suppressor in human and murine retinoblastoma development.Int J Cancer. 2008 May 1;122(9):2023-9. doi: 10.1002/ijc.23356. Int J Cancer. 2008. PMID: 18196575
-
The RB protein family in retinal development and retinoblastoma: new insights from new mouse models.Dev Neurosci. 2004;26(5-6):417-34. doi: 10.1159/000082284. Dev Neurosci. 2004. PMID: 15855771 Review.
-
Alternative reading frame supports an alternative model for retinoblastoma.Cell Cycle. 2003 Jan-Feb;2(1):27-30. doi: 10.4161/cc.2.1.252. Cell Cycle. 2003. PMID: 12695682 Review.
Cited by
-
PRELP Regulates Cell-Cell Adhesion and EMT and Inhibits Retinoblastoma Progression.Cancers (Basel). 2022 Oct 8;14(19):4926. doi: 10.3390/cancers14194926. Cancers (Basel). 2022. PMID: 36230849 Free PMC article.
-
Novel mutations in the RB1 gene from Chinese families with a history of retinoblastoma.Tumour Biol. 2015 Apr;36(4):2409-20. doi: 10.1007/s13277-014-2851-7. Epub 2014 Nov 27. Tumour Biol. 2015. PMID: 25424699
-
MFAP2 is overexpressed in gastric cancer and promotes motility via the MFAP2/integrin α5β1/FAK/ERK pathway.Oncogenesis. 2020 Feb 13;9(2):17. doi: 10.1038/s41389-020-0198-z. Oncogenesis. 2020. PMID: 32054827 Free PMC article.
-
Methylated promoters of genes encoding protocadherins as a new cancer biomarker family.Mol Biol Rep. 2012 Feb;39(2):1105-11. doi: 10.1007/s11033-011-0837-8. Epub 2011 May 21. Mol Biol Rep. 2012. PMID: 21598112
-
Kif14 overexpression accelerates murine retinoblastoma development.Int J Cancer. 2016 Oct 15;139(8):1752-8. doi: 10.1002/ijc.30221. Epub 2016 Jun 24. Int J Cancer. 2016. PMID: 27270502 Free PMC article.
References
-
- Gallie BL, Campbell C, Devlin H, Duckett A, Squire JA. Developmental basis of retinal-specific induction of cancer by RB mutation. Cancer Res. 1999;59:1731s–1735s. - PubMed
-
- Dimaras H, Coburn B, Pajovic S, Gallie BL. Loss of p75 neurotrophin receptor expression accompanies malignant progression to human and murine retinoblastoma. Mol Carcinog. 2006;45:333–343. - PubMed
-
- Dimaras H, Khetan V, Halliday W, Orlic M, Prigoda NL, et al. Loss of RB1 induces non-proliferative retinoma; increasing genomic instability correlates with progression to retinoblastoma. Hum Mol Genet. 2008. - PubMed
-
- Squire J, Gallie BL, Phillips RA. A detailed analysis of chromosomal changes in heritable and non-heritable retinoblastoma. Hum Genet. 1985;70:291–301. - PubMed
-
- Chen D, Gallie BL, Squire JA. Minimal regions of chromosomal imbalance in retinoblastoma detected by comparative genomic hybridization. Cancer Genet Cytogenet. 2001;129:57–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

