Calpain3 is expressed in a proteolitically active form in papillomavirus-associated urothelial tumors of the urinary bladder in cattle
- PMID: 20421977
- PMCID: PMC2858658
- DOI: 10.1371/journal.pone.0010299
Calpain3 is expressed in a proteolitically active form in papillomavirus-associated urothelial tumors of the urinary bladder in cattle
Abstract
Background: Calpain 3 (Capn3), also named p94, is a skeletal muscle tissue-specific protein known to be responsible for limb-girdle muscular dystrophy type 2A (LGMD2A). Recent experimental studies have hypothesized a pro-apoptotic role of Capn3 in some melanoma cell lines. So far the link between calpain3 and tumors comes from in vitro studies. The objective of this study was to describe Capn3 activation in naturally occurring urothelial tumors of the urinary bladder in cattle.
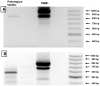
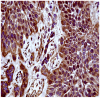
Methods and findings: Here we describe, for the first time in veterinary and comparative oncology, the activation of Capn3 in twelve urothelial tumor cells of the urinary bladder of cattle. Capn3 protein was initially identified with nanoscale liquid chromatography coupled with tandem mass spectrometry (nano LC-MS/MS) in a co-immunoprecipitation experiment on E2F3, known to be a transcription factor playing a crucial role in bladder carcinogenesis in humans. Capn3 expression was then confirmed by reverse transcription polymerase chain reaction (RT-PCR). Finally, the Ca(2+)-dependent proteolytic activity of Capn3 was assayed following ion exchange chromatography. Morphologically, Capn3 expression was documented by immunohistochemical methods. In fact numerous tumor cells showed an intracytoplasmic immunoreactivity, which was more rarely evident also at nuclear level. In urothelial tumors, bovine papillomavirus type 2 (BPV-2) DNA was amplified by PCR and the expression of E5 protein, the major oncogenic protein of BVP-2, was detected by western blotting, immunohistochemistry, and immunofluorescence. E2F3 overexpression and pRb protein downregulation were shown by western blotting.
Conclusion: The role of capn3 protein in urothelial cancer of the urinary bladder remains to be elucidated: further studies would be required to determine the precise function of this protease in tumor development and progression. However, we suggest that activated Capn3 may be involved in molecular pathways leading to the overexpression of E2F3, which in turn could be responsible for urothelial tumor cell proliferation also in cattle, though other mechanisms are likely to exist. If further studies corroborate the important role of Capn3 in urothelial tumors of the urinary bladder, cattle with urinary tumors may prove useful as animal model for bladder carcinogenesis.
Conflict of interest statement
Figures










Similar articles
-
Bovine papillomavirus type 2 (BPV-2) E5 oncoprotein binds to the subunit D of the V₁-ATPase proton pump in naturally occurring urothelial tumors of the urinary bladder of cattle.PLoS One. 2014 Feb 24;9(2):e88860. doi: 10.1371/journal.pone.0088860. eCollection 2014. PLoS One. 2014. PMID: 24586417 Free PMC article.
-
Productive infection of bovine papillomavirus type 2 in the urothelial cells of naturally occurring urinary bladder tumors in cattle and water buffaloes.PLoS One. 2013 May 7;8(5):e62227. doi: 10.1371/journal.pone.0062227. Print 2013. PLoS One. 2013. PMID: 23667460 Free PMC article.
-
Bovine Papillomavirus Type 13 Expression in the Urothelial Bladder Tumours of Cattle.Transbound Emerg Dis. 2016 Dec;63(6):628-634. doi: 10.1111/tbed.12322. Epub 2015 Jan 19. Transbound Emerg Dis. 2016. PMID: 25597262
-
Possible etiological association of ovine papillomaviruses with bladder tumors in cattle.Virus Res. 2023 Apr 15;328:199084. doi: 10.1016/j.virusres.2023.199084. Epub 2023 Mar 6. Virus Res. 2023. PMID: 36878382 Free PMC article. Review.
-
An eccentric calpain, CAPN3/p94/calpain-3.Biochimie. 2016 Mar;122:169-87. doi: 10.1016/j.biochi.2015.09.010. Epub 2015 Sep 10. Biochimie. 2016. PMID: 26363099 Review.
Cited by
-
Calpain-3 impairs cell proliferation and stimulates oxidative stress-mediated cell death in melanoma cells.PLoS One. 2015 Feb 6;10(2):e0117258. doi: 10.1371/journal.pone.0117258. eCollection 2015. PLoS One. 2015. PMID: 25658320 Free PMC article.
-
Association Between BoLA-DRB3.2 Polymorphism and Bovine Papillomavirus Infection for Bladder Tumor Risk in Podolica Cattle.Front Vet Sci. 2021 Jun 9;8:630089. doi: 10.3389/fvets.2021.630089. eCollection 2021. Front Vet Sci. 2021. PMID: 34179154 Free PMC article.
-
Bovine papillomavirus E5 and E7 oncoproteins in naturally occurring tumors: are two better than one?Infect Agent Cancer. 2013 Jan 9;8(1):1. doi: 10.1186/1750-9378-8-1. Infect Agent Cancer. 2013. PMID: 23302179 Free PMC article.
-
Molecular findings and virological assessment of bladder papillomavirus infection in cattle.Vet Q. 2024 Dec;44(1):1-7. doi: 10.1080/01652176.2024.2387072. Epub 2024 Aug 4. Vet Q. 2024. PMID: 39097798 Free PMC article.
-
Bovine papillomavirus type 2 (BPV-2) E5 oncoprotein binds to the subunit D of the V₁-ATPase proton pump in naturally occurring urothelial tumors of the urinary bladder of cattle.PLoS One. 2014 Feb 24;9(2):e88860. doi: 10.1371/journal.pone.0088860. eCollection 2014. PLoS One. 2014. PMID: 24586417 Free PMC article.
References
-
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. - PubMed
-
- Suzuchi K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53(Suppl. 1):S12–S18. - PubMed
-
- Stifanese R, Averna M, De Tullio R, Salamino F, Cantoni C, et al. Role of calpain-calpastatin system in the density-dependent growth arrest. Arch Biochem Biophys. 2008;479:145–152. - PubMed
-
- Demarchi F, Bertoli C, Copetti T, Eskelinen E-L, Schneider C. Calpain as a novel regulator of autophagosome formation. Autophagy. 2007;3:235–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

