Permanent occlusion of feeding arteries and draining veins in solid mouse tumors by vascular targeted photodynamic therapy (VTP) with Tookad
- PMID: 20421983
- PMCID: PMC2858664
- DOI: 10.1371/journal.pone.0010282
Permanent occlusion of feeding arteries and draining veins in solid mouse tumors by vascular targeted photodynamic therapy (VTP) with Tookad
Abstract
Background: Antiangiogenic and anti-vascular therapies present intriguing alternatives to cancer therapy. However, despite promising preclinical results and significant delays in tumor progression, none have demonstrated long-term curative features to date. Here, we show that a single treatment session of Tookad-based vascular targeted photodynamic therapy (VTP) promotes permanent arrest of tumor blood supply by rapid occlusion of the tumor feeding arteries (FA) and draining veins (DV), leading to tumor necrosis and eradication within 24-48 h.
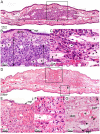
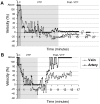
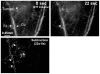
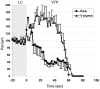
Methodology/principal findings: A mouse earlobe MADB106 tumor model was subjected to Tookad-VTP and monitored by three complementary, non-invasive online imaging techniques: Fluorescent intravital microscopy, Dynamic Light Scattering Imaging and photosensitized MRI. Tookad-VTP led to prompt tumor FA vasodilatation (a mean volume increase of 70%) with a transient increase (60%) in blood-flow rate. Rapid vasoconstriction, simultaneous blood clotting, vessel permeabilization and a sharp decline in the flow rates then followed, culminating in FA occlusion at 63.2 sec+/-1.5SEM. This blockage was deemed irreversible after 10 minutes of VTP treatment. A decrease in DV blood flow was demonstrated, with a slight lag from FA response, accompanied by frequent changes in flow direction before reaching a complete standstill. In contrast, neighboring, healthy tissue vessels of similar sizes remained intact and functional after Tookad-VTP.
Conclusion/significance: Tookad-VTP selectively targets the tumor feeding and draining vessels. To the best of our knowledge, this is the first mono-therapeutic modality that primarily aims at the larger tumor vessels and leads to high cure rates, both in the preclinical and clinical arenas.
Conflict of interest statement
Figures


References
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. - PubMed
-
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. - PubMed
-
- Dvorak HF. Angiogenesis: update 2005. J Thromb Haemost. 2005;3:1835–1842. - PubMed
-
- Cooney MM, van Heeckeren W, Bhakta S, Ortiz J, Remick SC. Drug insight: vascular disrupting agents and angiogenesis–novel approaches for drug delivery. Nat Clin Pract Oncol. 2006;3:682–692. - PubMed
-
- Tozer GM, Ameer-Beg SM, Baker J, Barber PR, Hill SA, et al. Intravital imaging of tumour vascular networks using multi-photon fluorescence microscopy. Adv Drug Deliv Rev. 2005;57:135–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

