Significant type I and type III collagen production from human periodontal ligament fibroblasts in 3D peptide scaffolds without extra growth factors
- PMID: 20421985
- PMCID: PMC2858666
- DOI: 10.1371/journal.pone.0010305
Significant type I and type III collagen production from human periodontal ligament fibroblasts in 3D peptide scaffolds without extra growth factors
Abstract
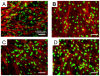
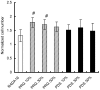
We here report the development of two peptide scaffolds designed for periodontal ligament fibroblasts. The scaffolds consist of one of the pure self-assembling peptide scaffolds RADA16 through direct coupling to short biologically active motifs. The motifs are 2-unit RGD binding sequence PRG (PRGDSGYRGDS) and laminin cell adhesion motif PDS (PDSGR). RGD and laminin have been previously shown to promote specific biological activities including periodontal ligament fibroblasts adhesion, proliferation and protein production. Compared to the pure RADA16 peptide scaffold, we here show that these designer peptide scaffolds significantly promote human periodontal ligament fibroblasts to proliferate and migrate into the scaffolds (for approximately 300 microm/two weeks). Moreover these peptide scaffolds significantly stimulated periodontal ligament fibroblasts to produce extracellular matrix proteins without using extra additional growth factors. Immunofluorescent images clearly demonstrated that the peptide scaffolds were almost completely covered with type I and type III collagens which were main protein components of periodontal ligament. Our results suggest that these designer self-assembling peptide nanofiber scaffolds may be useful for promoting wound healing and especially periodontal ligament tissue regeneration.
Conflict of interest statement
Figures




Similar articles
-
[Comparison of synthesizing Collagen I and III in periodontal ligament cells and gingival fibroblasts].Shanghai Kou Qiang Yi Xue. 2003 Jun;12(3):183-6. Shanghai Kou Qiang Yi Xue. 2003. PMID: 14661325 Chinese.
-
Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration.PLoS One. 2007 Feb 7;2(2):e190. doi: 10.1371/journal.pone.0000190. PLoS One. 2007. PMID: 17285144 Free PMC article.
-
The dynamic healing profile of human periodontal ligament stem cells: histological and immunohistochemical analysis using an ectopic transplantation model.J Periodontal Res. 2012 Aug;47(4):514-24. doi: 10.1111/j.1600-0765.2011.01463.x. Epub 2012 Feb 6. J Periodontal Res. 2012. PMID: 22308979
-
Biological aspects of periodontal tissue regeneration: cementogenesis and the induction of Sharpey's fibres.SADJ. 2013 Aug;68(7):304-6, 308-12, 314 passim. SADJ. 2013. PMID: 24133950 Review.
-
A Review of In Vivo and Clinical Studies Applying Scaffolds and Cell Sheet Technology for Periodontal Ligament Regeneration.Biomolecules. 2022 Mar 11;12(3):435. doi: 10.3390/biom12030435. Biomolecules. 2022. PMID: 35327627 Free PMC article. Review.
Cited by
-
"PP-type" self-assembling peptides with superior rheological properties.Nanoscale Adv. 2021 Sep 3;3(21):6056-6062. doi: 10.1039/d1na00534k. eCollection 2021 Oct 27. Nanoscale Adv. 2021. PMID: 36133953 Free PMC article.
-
Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering.Materials (Basel). 2018 Aug 20;11(8):1478. doi: 10.3390/ma11081478. Materials (Basel). 2018. PMID: 30127246 Free PMC article. Review.
-
A Comparative View on Human Somatic Cell Sources for iPSC Generation.Stem Cells Int. 2014;2014:768391. doi: 10.1155/2014/768391. Epub 2014 Nov 6. Stem Cells Int. 2014. PMID: 25431601 Free PMC article. Review.
-
Release systems based on self-assembling RADA16-I hydrogels with a signal sequence which improves wound healing processes.Sci Rep. 2023 Apr 18;13(1):6273. doi: 10.1038/s41598-023-33464-w. Sci Rep. 2023. PMID: 37072464 Free PMC article.
-
RGD- and VEGF-Mimetic Peptide Epitope-Functionalized Self-Assembling Peptide Hydrogels Promote Dentin-Pulp Complex Regeneration.Int J Nanomedicine. 2020 Sep 8;15:6631-6647. doi: 10.2147/IJN.S253576. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32982223 Free PMC article.
References
-
- Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnology. 2003;21:1171–1178. - PubMed
-
- Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, et al. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials. 1995;16:1385–1393. - PubMed
-
- Yang Y, Khoe U, Wang X, Horii A, Yokoi H, et al. Designer self-assembling peptide nanomaterials. Nano Today. 2009;4:193–210.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

