Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments
- PMID: 20422028
- PMCID: PMC2857745
- DOI: 10.1371/journal.pone.0010250
Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments
Abstract
Background: Toll-like receptors (TLRs) are among the first-line sentinels for immune detection and responsiveness to pathogens. The TLR2 subfamily of TLRs (TLR1, TLR2, TLR6) form heterodimers with each other and are thus able to recognize a broad range of components from several microbes such as yeast, Gram-positive bacteria and protozoa. Until now, TLR2 activation by bacterial ligands has long been associated with pro-inflammatory cytokines but not type I interferon responses.
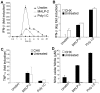
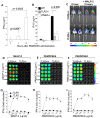
Methodology/principal findings: Using a variety of transgenic mice, here we provide in vivo and in vitro data showing that TLR2 activation does in fact induce interferon-beta and that this occurs via MyD88-IRF1 and -IRF7 pathways. Interestingly, by microscopy we demonstrate that although a cell surface receptor, TLR2 dependent induction of type I interferons occurs in endolysosomal compartments where it is translocated to upon ligand engagement. Furthermore, we could show that blocking receptor internalization or endolysosomal acidification inhibits the ability of TLR2 to trigger the induction type I interferon but not pro-inflammatory responses.
Conclusion/significance: The results indicate that TLR2 activation induces pro-inflammatory and type I interferon responses from distinct subcellular sites: the plasma membrane and endolysosomal compartments respectively. Apart from identifying and characterizing a novel pathway for induction of type I interferons, the present study offers new insights into how TLR signaling discriminates and regulates the nature of responses to be elicited against extracellular and endocytosed microbes. These findings may also have clinical implication. Excessive production of pro-inflammatory cytokines and type I IFNs following activation of TLRs is a central pathologic event in several hyper-inflammatory conditions. The discovery that the induction of pro-inflammatory and type I IFN responses can be uncoupled through pharmacological manipulation of endolysosomal acidification suggests new avenues for potential therapeutic intervention against inflammations and sepsis.
Conflict of interest statement
Figures




Similar articles
-
TRAM is required for TLR2 endosomal signaling to type I IFN induction.J Immunol. 2014 Dec 15;193(12):6090-102. doi: 10.4049/jimmunol.1401605. Epub 2014 Nov 10. J Immunol. 2014. PMID: 25385819 Free PMC article.
-
Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors.J Biol Chem. 2004 Apr 30;279(18):19008-17. doi: 10.1074/jbc.M311618200. Epub 2004 Feb 19. J Biol Chem. 2004. PMID: 14976215
-
Reprogramming of murine macrophages through TLR2 confers viral resistance via TRAF3-mediated, enhanced interferon production.PLoS Pathog. 2013;9(7):e1003479. doi: 10.1371/journal.ppat.1003479. Epub 2013 Jul 11. PLoS Pathog. 2013. PMID: 23853595 Free PMC article.
-
The role of Toll-like receptor 2 in microbial disease and immunity.Vaccine. 2003 Jun 1;21 Suppl 2:S55-60. doi: 10.1016/s0264-410x(03)00201-9. Vaccine. 2003. PMID: 12763684 Review.
-
Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses.Dermatology. 2005;211(3):193-8. doi: 10.1159/000087011. Dermatology. 2005. PMID: 16205063 Review.
Cited by
-
The Role of TLR2 in Infection and Immunity.Front Immunol. 2012 Apr 18;3:79. doi: 10.3389/fimmu.2012.00079. eCollection 2012. Front Immunol. 2012. PMID: 22566960 Free PMC article.
-
PRRs are watching you: Localization of innate sensing and signaling regulators.Virology. 2015 May;479-480:104-9. doi: 10.1016/j.virol.2015.02.051. Epub 2015 Mar 20. Virology. 2015. PMID: 25800355 Free PMC article. Review.
-
TLR signaling and DNA repair: are they associated?J Invest Dermatol. 2013 Feb;133(2):296-302. doi: 10.1038/jid.2012.288. Epub 2012 Aug 30. J Invest Dermatol. 2013. PMID: 22931928 Free PMC article. Review.
-
IRF1 regulates self-renewal and stress responsiveness to support hematopoietic stem cell maintenance.Sci Adv. 2023 Oct 27;9(43):eadg5391. doi: 10.1126/sciadv.adg5391. Epub 2023 Oct 27. Sci Adv. 2023. PMID: 37889967 Free PMC article.
-
Toll-Like Receptor (TLR) Signaling Enables Cyclic GMP-AMP Synthase (cGAS) Sensing of HIV-1 Infection in Macrophages.mBio. 2021 Dec 21;12(6):e0281721. doi: 10.1128/mBio.02817-21. Epub 2021 Nov 30. mBio. 2021. PMID: 34844429 Free PMC article.
References
-
- Kenny EF, O'Neill LA. Signalling adaptors used by Toll-like receptors: an update. Cytokine. 2008;43:342–349. - PubMed
-
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. - PubMed
-
- Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–15323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

