Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells
- PMID: 20422040
- PMCID: PMC2857883
- DOI: 10.1371/journal.pone.0010237
Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells
Abstract
Background: The epithelial cell response to stress involves the transmission of signals between contiguous cells that can be visualized as a calcium wave. In some cell types, this wave is dependent on the release of extracellular trinucleotides from injured cells. In particular, extracellular ATP has been reported to be critical for the epithelial cell response to stress and has recently been shown to be upregulated in tumors in vivo.
Methodology/principal findings: Here, we identify stanniocalcin-1 (STC1), a secreted pleiotrophic protein, as a critical mediator of calcium wave propagation in monolayers of pulmonary (A549) and prostate (PC3) epithelial cells. Addition of STC1 enhanced and blocking STC1 decreased the distance traveled by an extracellular ATP-dependent calcium wave. The same effects were observed when calcium was stimulated by the addition of exogenous ATP. We uncover a positive feedback loop in which STC1 promotes the release of ATP from cells in vitro and in vivo.
Conclusions/significance: The results indicated that STC1 plays an important role in the early response to mechanical injury by epithelial cells by modulating signaling of extracellular ATP. This is the first report to describe STC1 as a modulator or purinergic receptor signaling.
Conflict of interest statement
Figures
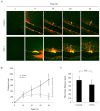


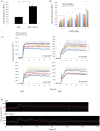
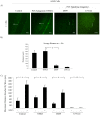

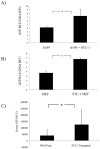
Similar articles
-
Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides.J Physiol. 2007 Oct 15;584(Pt 2):419-35. doi: 10.1113/jphysiol.2007.133314. Epub 2007 Aug 16. J Physiol. 2007. PMID: 17702822 Free PMC article.
-
Cancer Stem Cell Formation Induced and Regulated by Extracellular ATP and Stanniocalcin-1 in Human Lung Cancer Cells and Tumors.Int J Mol Sci. 2022 Nov 25;23(23):14770. doi: 10.3390/ijms232314770. Int J Mol Sci. 2022. PMID: 36499099 Free PMC article.
-
Extracellular ATP induces cytoplasmic and nuclear Ca2+ transients via P2Y2 receptor in human biliary epithelial cancer cells (Mz-Cha-1).Anticancer Res. 2012 Sep;32(9):3759-67. Anticancer Res. 2012. PMID: 22993317
-
ATP synthesis and storage.Purinergic Signal. 2012 Sep;8(3):343-57. doi: 10.1007/s11302-012-9305-8. Epub 2012 Apr 12. Purinergic Signal. 2012. PMID: 22528680 Free PMC article. Review.
-
Calcium wave propagation during cell extrusion.Curr Opin Cell Biol. 2022 Jun;76:102083. doi: 10.1016/j.ceb.2022.102083. Epub 2022 Apr 26. Curr Opin Cell Biol. 2022. PMID: 35487153 Review.
Cited by
-
Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1.Mol Ther. 2012 Feb;20(2):417-23. doi: 10.1038/mt.2011.259. Epub 2011 Dec 6. Mol Ther. 2012. PMID: 22146344 Free PMC article.
-
Calcium Oscillatory Behavior and Its Possible Role during Wound Healing in Bovine Corneal Endothelial Cells in Culture.Biomed Res Int. 2019 Feb 21;2019:8647121. doi: 10.1155/2019/8647121. eCollection 2019. Biomed Res Int. 2019. PMID: 30915363 Free PMC article.
-
Stimulation of Local Cytosolic Calcium Release by Photothermal Heating for Studying Intra- and Intercellular Calcium Waves.Adv Mater. 2021 Jun;33(24):e2008261. doi: 10.1002/adma.202008261. Epub 2021 May 5. Adv Mater. 2021. PMID: 33949733 Free PMC article.
-
Stanniocalcin-1 is induced by hypoxia inducible factor in rat alveolar epithelial cells.Biochem Biophys Res Commun. 2014 Oct 3;452(4):1091-7. doi: 10.1016/j.bbrc.2014.09.060. Epub 2014 Sep 22. Biochem Biophys Res Commun. 2014. PMID: 25251473 Free PMC article.
-
Purinergic signalling and cancer.Purinergic Signal. 2013 Dec;9(4):491-540. doi: 10.1007/s11302-013-9372-5. Purinergic Signal. 2013. PMID: 23797685 Free PMC article. Review.
References
-
- Wagner GF, Hampong M, Park CM, Copp DH. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol. 1986;63:481–491. - PubMed
-
- Lafeber FP, Herrmann-Erlee MP, Flik G, Wendelaar Bonga SE. Rainbow trout hypocalcin stimulates bone resorption in embryonic mouse calvaria in vitro in a PTH-like fashion. J Exp Biol. 1989;143:165–175. - PubMed
-
- Wagner GF, Vozzolo BL, Jaworski E, Haddad M, Kline RL, et al. Human stanniocalcin inhibits renal phosphate excretion in the rat. J Bone Miner Res. 1997;12:165–171. - PubMed
-
- Madsen KL, Tavernini MM, Yachimec C, Mendrick DL, Alfonso PJ, et al. Stanniocalcin: a novel protein regulating calcium and phosphate transport across mammalian intestine. Am J Physiol. 1998;274:G96–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

