Immunoregulatory actions of epithelial cell PPAR gamma at the colonic mucosa of mice with experimental inflammatory bowel disease
- PMID: 20422041
- PMCID: PMC2857885
- DOI: 10.1371/journal.pone.0010215
Immunoregulatory actions of epithelial cell PPAR gamma at the colonic mucosa of mice with experimental inflammatory bowel disease
Abstract
Background: Peroxisome proliferator-activated receptors are nuclear receptors highly expressed in intestinal epithelial cells (IEC) and immune cells within the gut mucosa and are implicated in modulating inflammation and immune responses. The objective of this study was to investigate the effect of targeted deletion of PPAR gamma in IEC on progression of experimental inflammatory bowel disease (IBD).
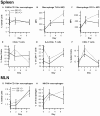
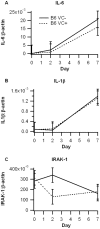
Methodology/principal findings: In the first phase, PPAR gamma flfl; Villin Cre- (VC-) and PPAR gamma flfl; Villin Cre+ (VC+) mice in a mixed FVB/C57BL/6 background were challenged with 2.5% dextran sodium sulfate (DSS) in drinking water for 0, 2, or 7 days. VC+ mice express a transgenic recombinase under the control of the Villin-Cre promoter that causes an IEC-specific deletion of PPAR gamma. In the second phase, we generated VC- and VC+ mice in a C57BL/6 background that were challenged with 2.5% DSS. Mice were scored on disease severity both clinically and histopathologically. Flow cytometry was used to phenotypically characterize lymphocyte and macrophage populations in blood, spleen and mesenteric lymph nodes. Global gene expression analysis was profiled using Affymetrix microarrays. The IEC-specific deficiency of PPAR gamma in mice with a mixed background worsened colonic inflammatory lesions, but had no effect on disease activity (DAI) or weight loss. In contrast, the IEC-specific PPAR gamma null mice in C57BL/6 background exhibited more severe inflammatory lesions, DAI and weight loss in comparison to their littermates expressing PPAR gamma in IEC. Global gene expression profiling revealed significantly down-regulated expression of lysosomal pathway genes and flow cytometry results demonstrated suppressed production of IL-10 by CD4+ T cells in mesenteric lymph nodes (MLN) of IEC-specific PPAR gamma null mice.
Conclusions/significance: Our results demonstrate that adequate expression of PPAR gamma in IEC is required for the regulation of mucosal immune responses and prevention of experimental IBD, possibly by modulation of lysosomal and antigen presentation pathways.
Conflict of interest statement
Figures






Similar articles
-
Expression of PPAR γ in intestinal epithelial cells is dispensable for the prevention of colitis by dietary abscisic acid.ESPEN J. 2012 Oct 1;7(5):e189-e195. doi: 10.1016/j.clnme.2012.07.002. ESPEN J. 2012. PMID: 23814701 Free PMC article.
-
The role of T cell PPAR gamma in mice with experimental inflammatory bowel disease.BMC Gastroenterol. 2010 Jun 10;10:60. doi: 10.1186/1471-230X-10-60. BMC Gastroenterol. 2010. PMID: 20537136 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
The acetylome regulators Hdac1 and Hdac2 differently modulate intestinal epithelial cell dependent homeostatic responses in experimental colitis.Am J Physiol Gastrointest Liver Physiol. 2014 Apr 1;306(7):G594-605. doi: 10.1152/ajpgi.00393.2013. Epub 2014 Feb 13. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24525021
-
On cell death in the intestinal epithelium and its impact on gut homeostasis.Curr Opin Gastroenterol. 2018 Nov;34(6):413-419. doi: 10.1097/MOG.0000000000000481. Curr Opin Gastroenterol. 2018. PMID: 30169459 Free PMC article. Review.
Cited by
-
PPARγ in Inflammatory Bowel Disease.PPAR Res. 2012;2012:620839. doi: 10.1155/2012/620839. Epub 2012 Sep 10. PPAR Res. 2012. PMID: 22997506 Free PMC article.
-
Innate immune signalling at the intestinal epithelium in homeostasis and disease.EMBO Rep. 2012 Aug;13(8):684-98. doi: 10.1038/embor.2012.96. Epub 2012 Jul 17. EMBO Rep. 2012. PMID: 22801555 Free PMC article. Review.
-
Expression of PPAR γ in intestinal epithelial cells is dispensable for the prevention of colitis by dietary abscisic acid.ESPEN J. 2012 Oct 1;7(5):e189-e195. doi: 10.1016/j.clnme.2012.07.002. ESPEN J. 2012. PMID: 23814701 Free PMC article.
-
Endocrine and metabolic manifestations in inflammatory bowel disease.Ann Gastroenterol. 2012;25(1):37-44. Ann Gastroenterol. 2012. PMID: 24714153 Free PMC article. Review.
-
Dietary α-eleostearic acid ameliorates experimental inflammatory bowel disease in mice by activating peroxisome proliferator-activated receptor-γ.PLoS One. 2011;6(8):e24031. doi: 10.1371/journal.pone.0024031. Epub 2011 Aug 31. PLoS One. 2011. PMID: 21904603 Free PMC article.
References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat Res. 2009;669:1–7. - PubMed
-
- Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178:2940–2949. - PubMed
-
- Chen L, Bush CR, Necela BM, Su W, Yanagisawa M, et al. RS5444, a novel PPARgamma agonist, regulates aspects of the differentiated phenotype in nontransformed intestinal epithelial cells. Mol Cell Endocrinol. 2006;251:17–32. - PubMed
-
- Bassaganya-Riera J, Hontecillas R. CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Clin Nutr. 2006;25:454–465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

