Phosphorylation of AATYK1 by Cdk5 suppresses its tyrosine phosphorylation
- PMID: 20422042
- PMCID: PMC2857886
- DOI: 10.1371/journal.pone.0010260
Phosphorylation of AATYK1 by Cdk5 suppresses its tyrosine phosphorylation
Abstract
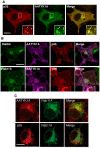
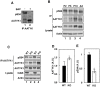
Apoptosis-associated tyrosine kinase 1 (AATYK1), a novel serine/threonine kinase that is highly expressed in the brain, is involved in neurite extension and apoptosis of cerebellar granule neurons; however, its precise function remains unknown. In this study, we investigated the interaction of AATYK1A with Cyclin-dependent kinase 5 (Cdk5)/p35, a proline-directed protein kinase that is predominantly expressed in neurons. AATYK1A bound to the p35 activation subunit of Cdk5 in cultured cells and in mouse brains and colocalized with p35 on endosomes in COS-7 cells. AATYK1A was phosphorylated at Ser34 by Cdk5/p35 in vitro, in cultured neurons and in mouse brain. In PC12D cells, Ser34 phosphorylation increased after treatment with nerve growth factor and phosphorylated AATYK1A accumulated in growth cones of PC12D cells. Ser34 phosphorylation suppressed the tyrosine phosphorylation of AATYK1A by Src family kinases. These results suggest a possibility that AATYK1A plays a role in early to recycling endosomes and its function is regulated by phosphorylation with Cdk5 or Src-family kinases.
Conflict of interest statement
Figures




Similar articles
-
Palmitoylation-dependent endosomal localization of AATYK1A and its interaction with Src.Genes Cells. 2008 Sep;13(9):949-64. doi: 10.1111/j.1365-2443.2008.01219.x. Epub 2008 Aug 7. Genes Cells. 2008. PMID: 18691334
-
AATYK1A phosphorylation by Cdk5 regulates the recycling endosome pathway.Genes Cells. 2010 Jun;15(7):783-97. doi: 10.1111/j.1365-2443.2010.01419.x. Epub 2010 Jun 11. Genes Cells. 2010. PMID: 20553326
-
Phosphorylation of cyclin-dependent kinase 5 (Cdk5) at Tyr-15 is inhibited by Cdk5 activators and does not contribute to the activation of Cdk5.J Biol Chem. 2014 Jul 11;289(28):19627-36. doi: 10.1074/jbc.M113.501148. Epub 2014 May 28. J Biol Chem. 2014. PMID: 24872417 Free PMC article.
-
The regulation of cyclin-dependent kinase 5 activity through the metabolism of p35 or p39 Cdk5 activator.Neurosignals. 2003 Sep-Oct;12(4-5):221-9. doi: 10.1159/000074624. Neurosignals. 2003. PMID: 14673209 Review.
-
The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer's pathology.Eur J Biochem. 2001 Mar;268(6):1518-27. doi: 10.1046/j.1432-1033.2001.02024.x. Eur J Biochem. 2001. PMID: 11248668 Review.
Cited by
-
Phosphorylation of drebrin by cyclin-dependent kinase 5 and its role in neuronal migration.PLoS One. 2014 Mar 17;9(3):e92291. doi: 10.1371/journal.pone.0092291. eCollection 2014. PLoS One. 2014. PMID: 24637538 Free PMC article.
-
Transcriptomic Analysis of mRNAs in Human Monocytic Cells Expressing the HIV-1 Nef Protein and Their Exosomes.Biomed Res Int. 2015;2015:492395. doi: 10.1155/2015/492395. Epub 2015 Apr 15. Biomed Res Int. 2015. PMID: 25961023 Free PMC article.
-
Identification of a factor controlling lysosomal homeostasis using a novel lysosomal trafficking probe.Sci Rep. 2019 Aug 12;9(1):11635. doi: 10.1038/s41598-019-48131-2. Sci Rep. 2019. PMID: 31406169 Free PMC article.
-
LMTK1 regulates dendritic formation by regulating movement of Rab11A-positive endosomes.Mol Biol Cell. 2014 Jun;25(11):1755-68. doi: 10.1091/mbc.E14-01-0675. Epub 2014 Mar 26. Mol Biol Cell. 2014. PMID: 24672056 Free PMC article.
-
The lemur tail kinase family in neuronal function and disfunction in neurodegenerative diseases.Cell Mol Life Sci. 2024 Nov 9;81(1):447. doi: 10.1007/s00018-024-05480-0. Cell Mol Life Sci. 2024. PMID: 39520508 Free PMC article. Review.
References
-
- Tomomura M, Morita N, Yoshikawa F, Konishi A, Akiyama H, et al. Structural and functional analysis of the apoptosis-associated tyrosine kinase (AATYK) family. Neuroscience. 2007;148:510–521. - PubMed
-
- Gaozza E, Baker SJ, Vora RK, Reddy EP. AATYK: a novel tyrosine kinase induced during growth arrest and apoptosis of myeloid cells. Oncogene. 1997;15:3127–3135. - PubMed
-
- Raghunath M, Patti R, Bannerman P, Lee CM, Baker S, et al. A novel kinase, AATYK induces and promotes neuronal differentiation in a human neuroblastoma (SH-SY5Y) cell line. Brain Res Mol Brain Res. 2000;77:151–162. - PubMed
-
- Wang H, Brautigan DL. A novel transmembrane Ser/Thr kinase complexes with protein phosphatase-1 and inhibitor-2. J Biol Chem. 2002;277:49605–49612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

