Development and evaluation of hydrophilic colloid matrix of famotidine tablets
- PMID: 20422332
- PMCID: PMC2902325
- DOI: 10.1208/s12249-010-9427-7
Development and evaluation of hydrophilic colloid matrix of famotidine tablets
Abstract
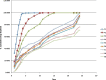
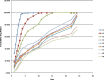
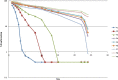
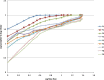
The objective of the present study was to develop a once-daily sustained-release (SR) matrix tablet of famotidine. Nine different formulations (F1-F9) were prepared by direct compression method using Avicel PH101 as filler/binder in the range of 41-27% in F1-F3, 18-22% in F4-F7, and 16-18% in F8-F9 and hydroxypropyl methylcellulose (4,000 cps) as hydrophilic matrix was used in F1-F3 from 19% to 30%, around 40% in F4-F7, and 42-45% in F8-F9. Talc and Aerosil were added in the ratio of 0.7-1.2%. The tablets were subjected to various physical parameters including weight variation test, hardness, thickness, diameter, friability, and in vitro release studies. Assay was also performed according to the USP 30 NF 25 procedure. The results of the physical parameters and assay were found to be within the acceptable range. In vitro dissolution results indicated that formulation F4-F7, having around 40% of rate control polymer, produced a SR pattern throughout 24 h. F1-F3 showed drug release at a faster rate, while F8-F9 released much slower, i.e., <80% in 24 h. Model-dependent and model-independent methods were used for data analysis and the best results were observed for F4 in zero order (r(2) = 0.984) and F6 in Korsmeyer and Higuchi (r(2) = 0.992 and 0.988). The parameter n indicated anomalous diffusion, while beta in Weibull showed a parabolic curve with higher initial slope. The f(2) similarity test was performed taking F4 as a reference formulation. Only the F5-F7 formulations were similar to the reference formulation F4. The mean dissolution time was around 10 h for the successful formulation.
Figures




Similar articles
-
Design, development and in-vitro evaluation of metoprolol tartrate tablets containing xanthan-tragacanth.Acta Pol Pharm. 2010 Sep-Oct;67(5):517-22. Acta Pol Pharm. 2010. PMID: 20873420
-
Formulation development and in-vitro evaluation of gastroretentive drug delivery system of loxoprofen sodium: A natural excipients based approach.Pak J Pharm Sci. 2021 Jan;34(1):57-63. Pak J Pharm Sci. 2021. PMID: 34248003
-
In vitro release of ketoprofen from hydrophilic matrix tablets containing cellulose polymer mixtures.Drug Dev Ind Pharm. 2013 Nov;39(11):1651-62. doi: 10.3109/03639045.2012.729146. Epub 2012 Oct 24. Drug Dev Ind Pharm. 2013. PMID: 23094867
-
Formulation development and evaluation of Diltiazem HCl sustained release matrix tablets using HPMC K4M and K100M.Pak J Pharm Sci. 2013 Jul;26(4):653-63. Pak J Pharm Sci. 2013. PMID: 23811439
-
Formulation and in vitro evaluation of sustained release matrix tablets using cross-linked natural gum.Pak J Pharm Sci. 2017 Mar;30(2):355-362. Pak J Pharm Sci. 2017. PMID: 28649056
Cited by
-
Application of Micellar Extraction for Isolation of Famotidine from Aqueous Samples Prior to its Chromatographic Determination.J Surfactants Deterg. 2017;20(6):1401-1409. doi: 10.1007/s11743-017-2003-3. Epub 2017 Aug 12. J Surfactants Deterg. 2017. PMID: 29200812 Free PMC article.
-
Successful Application of Alpha Lipoic Acid Niosomal Formulation in Cerebral Ischemic Reperfusion Injury in Rat Model.Adv Pharm Bull. 2022 May;12(3):541-549. doi: 10.34172/apb.2022.058. Epub 2021 Sep 12. Adv Pharm Bull. 2022. PMID: 35935040 Free PMC article.
-
Manipulation of the Glass Transition Properties of a High-Solid System Made of Acrylic Acid-N,N'-Methylenebisacrylamide Copolymer Grafted on Hydroxypropyl Methyl Cellulose.Int J Mol Sci. 2021 Mar 6;22(5):2682. doi: 10.3390/ijms22052682. Int J Mol Sci. 2021. PMID: 33800956 Free PMC article.
-
Aceclofenac fast dispersible tablet formulations: Effect of different concentration levels of Avicel PH102 on the compactional, mechanical and drug release characteristics.PLoS One. 2020 Feb 12;15(2):e0223201. doi: 10.1371/journal.pone.0223201. eCollection 2020. PLoS One. 2020. PMID: 32050259 Free PMC article.
-
Sustainable Drug Delivery of Famotidine Using Chitosan-Functionalized Graphene Oxide as Nanocarrier.Glob Chall. 2019 Aug 14;3(10):1900002. doi: 10.1002/gch2.201900002. eCollection 2019 Oct. Glob Chall. 2019. PMID: 31592120 Free PMC article.
References
-
- Dollery C. Therapeutic drugs. 2. Edinburgh: Churchill Livingstone; 1999.
-
- Pepsid. Electronic medicines compendium. Aventis Pharma Ltd. http://emc.medicines.org.uk/emc/assets/c/html/display.doc. Accessed 01 Aug 2003.
-
- Alderman DA. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int J Pharm Technol Prod Manuf. 1984;5:1–9.
-
- Reza MS, Quadir MA, Haid SS. Comparative evaluation of plastic hydrophobic and hydrophilic polymers as matrices for controlled release drug delivery. J Pharm Pharm Sci. 2003;6(2):274–291. - PubMed
-
- Gothoskar AV. Study of effect of polymer viscosity and polymer: excipient ratio on drug-release patterns from swellable matrices. Drug Delivery Technology. 2005;5:1–7.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

