Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease
- PMID: 20422735
- PMCID: PMC3018745
- DOI: 10.1097/fjc.0b013e3181d89670
Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease
Abstract
Vascular disease in hypertension and diabetes is associated with increased oxidants. The oxidants arise from NADPH oxidase, xanthine oxidase, and mitochondria. Superoxide anion and hydrogen peroxide are produced by both leukocytes and vascular cells. Nitric oxide is produced in excess by inducible nitric oxide synthase, and the potent oxidant, peroxynitrite, is formed from superoxide and nitric oxide. The damage to proteins caused by oxidants is selective, affecting specific oxidant-sensitive amino acid residues. With some important vascular proteins, for example, endothelial nitric oxide synthase, prostacycline synthase, and superoxide dismutase, oxidation of a single susceptible amino acid inactivates the enzyme. The beneficial effects of antioxidants, at least in animal models of hypertension and diabetes, can in part be ascribed to protection of these and other proteins. Mutant proteins lacking their reactive constituent can recapitulate some disease phenotypes suggesting a pathogenic role of the oxidation. Thus, many of the shared functional abnormalities of hypertensive and diabetic blood vessels may be caused by oxidants. Although studies using antioxidants have failed in patients, the successful treatment of vascular disease with HMG-CoA reductase inhibitors, thromboxane A2 antagonists, and polyphenols may depend on their anti-inflammatory effects and ability to decrease production of damaging oxidants.
Figures


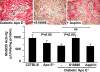
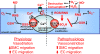

References
-
- Contreras F, Rivera M, Vasquez J, De la Parte MA, Velasco M. Diabetes and hypertension physiopathology and therapeutics. J Hum Hypertens. 2000;14(Suppl 1):S26–S31. - PubMed
-
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–44. - PubMed
-
- Savoia C, Schiffrin EL. Inhibition of the renin angiotensin system: implications for the endothelium. Curr Diab Rep. 2006;6(4):274–8. - PubMed
-
- Adachi T, Schoneich C, Cohen RA. S-glutathiolation in redox-sensitive signaling. Drug Discov Today. 2005;2(1):39–46.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

