Characterization of glycosaminoglycans by 15N NMR spectroscopy and in vivo isotopic labeling
- PMID: 20423049
- PMCID: PMC2884402
- DOI: 10.1021/ac1001383
Characterization of glycosaminoglycans by 15N NMR spectroscopy and in vivo isotopic labeling
Erratum in
- Anal Chem. 2011 Feb 1;83(3):1162
Abstract
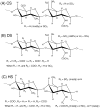
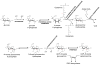
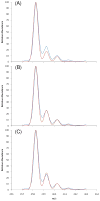
Characterization of glycosaminoglycans (GAGs), including chondroitin sulfate (CS), dermatan sulfate (DS), and heparan sulfate (HS), is important in developing an understanding of cellular function and in assuring quality of preparations destined for biomedical applications. While use of (1)H and (13)C NMR spectroscopy has become common in characterization of these materials, spectra are complex and difficult to interpret when a more heterogeneous GAG type or a mixture of several types is present. Herein a method based on (1)H-(15)N two-dimensional NMR experiments is described. The (15)N- and (1)H-chemical shifts of amide signals from (15)N-containing acetylgalactosamines in CSs are shown to be quite sensitive to the sites of sulfation (4-, 6-, or 4,6-) and easily distinguishable from those of DS. The amide signals from residual (15)N-containing acetylglucosamines in HS are shown to be diagnostic of the presence of these GAG components as well. Most data were collected at natural abundance of (15)N despite its low percentage. However enrichment of the (15)N-content in GAGs using metabolic incorporation from (15)N-glutamine added to cell culture media is also demonstrated and used to distinguish metabolic states in different cell types.
Figures




References
-
- Heino J, Kapyla J. Curr Pharm Des. 2009;15:1309–1317. - PubMed
-
- Yu F, Wolff JJ, Amster IJ, Prestegard JH. J Am Chem Soc. 2007;129:13288–13297. - PubMed
-
- Gandhi NS, Mancera RL. Chem Biol Drug Des. 2008;72:455–482. - PubMed
-
- Iozzo RV. Annu Rev Biochem. 1998;67:609–652. - PubMed
-
- Beaulieu JF, Vachon PH, Chartrand S. Anat Embryol. 1991;183:363–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

