Regulation of the protein C anticoagulant and antiinflammatory pathways
- PMID: 20423310
- PMCID: PMC2904412
- DOI: 10.2174/092986710791233706
Regulation of the protein C anticoagulant and antiinflammatory pathways
Abstract
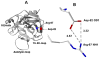
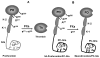
Protein C is a vitamin K-dependent anticoagulant serine protease zymogen in plasma which upon activation by the thrombin-thrombomodulin complex down-regulates the coagulation cascade by degrading cofactors Va and VIIIa by limited proteolysis. In addition to its anticoagulant function, activated protein C (APC) also binds to endothelial protein C receptor (EPCR) in lipid-rafts/caveolar compartments to activate protease- activated receptor 1 (PAR-1) thereby eliciting antiinflammatory and cytoprotective signaling responses in endothelial cells. These properties have led to FDA approval of recombinant APC as a therapeutic drug for severe sepsis. The mechanism by which APC selects its substrates in the anticoagulant and antiinflammatory pathways is not well understood. Recent structural and mutagenesis data have indicated that basic residues of three exposed surface loops known as 39-loop (Lys-37, Lys-38, and Lys-39), 60-loop (Lys-62, Lys- 63, and Arg-67), and 70-80-loop (Arg-74, Arg-75, and Lys-78) (chymotrypsin numbering) constitute an anion binding exosite in APC that interacts with the procoagulant cofactors Va and VIIIa in the anticoagulant pathway. Furthermore, two negatively charged residues on the opposite side of the active-site of APC on a helical structure have been demonstrated to determine the specificity of the PAR-1 recognition in the cytoprotective pathway. This article will review the mechanism by which APC exerts its proteolytic function in two physiologically inter-related pathways and how the structure- function insights into determinants of the specificity of APC interaction with its substrates in two pathways can be utilized to tinker with the structure of the molecule to obtain APC derivatives with potentially improved therapeutic profiles.
Figures

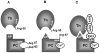

References
-
- Esmon CT. Molecular events that control the protein C anticoagulant pathway. Thromb Haemost. 1993;70:1–5. - PubMed
-
- Stenflo J. Structure-function relationships of epidermal growth factor modules in vitamin K-dependent clotting factors. Blood. 1991;78:1637–1651. - PubMed
-
- Walker FJ, Fay PJ. Regulation of blood coagulation by the protein C system. FASEB J. 1992;6:2561–2567. - PubMed
-
- Dahlbäck B. Protein S and C4b-binding protein: Components involved in the regulation of the protein C anticoagulant system. Thromb Haemostas. 1991;66:49–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

