Sonographic evaluation of pediatric localized scleroderma: preliminary disease assessment measures
- PMID: 20423513
- PMCID: PMC2878299
- DOI: 10.1186/1546-0096-8-14
Sonographic evaluation of pediatric localized scleroderma: preliminary disease assessment measures
Abstract
Background: Our earlier work in the ultrasonograpy of localized scleroderma (LS) suggests that altered levels of echogenicity and vascularity can be associated with disease activity. Utrasound is clinically benign and readily available, but can be limited by operator dependence. We present our efforts to standardize image acquisition and interpretation of pediatric LS to better evaluate the correlation between specific sonographic findings and disease activity.
Methods: Several meetings have been held among our multi-center group (LOCUS) to work towards standardizing sonographic technique and image interpretation. Demonstration and experience in image acquisition were conducted at workshop meetings. Following meetings in 2007, an ultrasound measure was developed to standardize evaluation of differences in echogenicity and vascularity. Based upon our initial observations, we have labeled this an ultrasound disease activity measure. This preliminary measure was subsequently evaluated on over 180 scans of pediatric LS lesions. This review suggested that scoring levels should be expanded to better capture the range of observed differences. The revised levels and their definitions were formulated at a February 2009 workshop meeting. We have also developed assessments for scoring changes in tissue thickness and lesion size to better determine if these parameters aid evaluation of disease state.
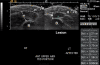
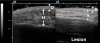
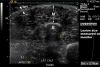
Results: We have standardized our protocol for acquiring ultrasound images of pediatric LS lesions. A wide range of sonographic differences has been seen in the dermis, hypodermis, and deep tissue layers of active lesions. Preliminary ultrasound assessments have been generated. The disease activity measure scores for altered levels of echogenicity and vascularity in the lesion, and other assessments score for differences in lesion tissue layer thickness and changes in lesion size.
Conclusions: We describe the range of sonographic differences found in pediatric LS, and present our efforts to standardize ultrasound acquisition and image interpretation for this disease. We present ultrasound measures that may aid evaluation of disease state. These assessments should be considered a work in progress, whose purpose is to facilitate further study in this area. More studies are needed to assess their validity and reliability.
Figures





References
-
- Jaworsky C. In: Lever's Histopathology of the Skin. Elder D, Elenitsas R, Johnson B, et al, editor. Lippincott Williams & Wilkins: Philadelphia; 2005. Connective Tissue Disease; pp. 311–2.
-
- Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, Espada G, Corona F, Mukamel M, Vesely R, Musiej-Nowakowska E, Chaitow J, Ros J, Apaz M, Gerloni V, Mazur-Zielinska H, Nielsen S, Ullman S, Horneff G, Wouters C, Martini G, Cimaz R, Laxer R, Athreya B. Localized Scleroderma in Childhood is Not Just a Skin Disease. Arthritis Rheum. 2005;52:2873–81. doi: 10.1002/art.21264. - DOI - PubMed
-
- Medsger T Jr, Silman A, Steen V, Black C, Akesson A, Bacon P, Harris C, Jablonska S, Jayson M, Jimenez S, Kreig T, Leroy E, Maddison P, Russell M, Schachter R, Wollheim F, Zacharaie H. A Disease Severity Scale for Systemic Sclerosis: Development and Testing. J Rheumatol. 1999;26:2159–67. - PubMed
LinkOut - more resources
Full Text Sources

