Locomotor and stress responses to nicotine differ in adolescent and adult rats
- PMID: 20423718
- PMCID: PMC2894530
- DOI: 10.1016/j.pbb.2010.04.010
Locomotor and stress responses to nicotine differ in adolescent and adult rats
Abstract
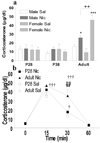
Since adolescence is a critical period for the initiation of tobacco use, we have systematically compared behavioral and endocrine responses to nicotine in Sprague-Dawley rats of both sexes at early adolescence (postnatal day (P) 28), mid- adolescence (P38) and adulthood (P90). Locomotion and center time in a novel open field were evaluated for 30min following intravenous injection of saline or nicotine (60microg/kg), followed by measurement of plasma corticosterone. Complex age and sex differences in behavioral and endocrine response were observed, which were dependent on the functional endpoint examined. Whereas there were age differences in nicotine effects on all functional measures, sex differences were largely restricted to adult stress-related corticosterone and center-time responses. Although significant drug effects were detected at P28 and P90, there was no effect of nicotine at P38 on any measure examined. In saline-treated males, but not females, there were significant positive correlations across age between initial ambulatory counts and both initial vertical counts and total center time. Nicotine treatment increased correlations in both sexes, and yielded a significant negative interaction between initial ambulatory counts and plasma corticosterone. The unique responses of adolescents to nicotine are consistent with an immature function of nicotinic acetylcholine receptors at this age.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats.Horm Behav. 2004 Nov;46(4):458-66. doi: 10.1016/j.yhbeh.2004.05.004. Horm Behav. 2004. PMID: 15465532
-
Nicotine-induced plasma corticosterone is attenuated by social interactions in male and female adolescent rats.Pharmacol Biochem Behav. 2011 Nov;100(1):1-7. doi: 10.1016/j.pbb.2011.07.005. Epub 2011 Jul 18. Pharmacol Biochem Behav. 2011. PMID: 21782841 Free PMC article.
-
Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats.Neuropsychopharmacology. 2007 Sep;32(9):2025-35. doi: 10.1038/sj.npp.1301327. Epub 2007 Feb 7. Neuropsychopharmacology. 2007. PMID: 17287824
-
Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats.Pharmacol Biochem Behav. 2007 Feb;86(2):297-305. doi: 10.1016/j.pbb.2006.10.002. Epub 2006 Dec 1. Pharmacol Biochem Behav. 2007. PMID: 17141304
-
Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats.Addict Biol. 2008 Mar;13(1):63-9. doi: 10.1111/j.1369-1600.2007.00080.x. Epub 2007 Sep 11. Addict Biol. 2008. PMID: 17850415
Cited by
-
Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence.Neurobiol Stress. 2016 Aug 29;6:31-43. doi: 10.1016/j.ynstr.2016.08.003. eCollection 2017 Feb. Neurobiol Stress. 2016. PMID: 28229107 Free PMC article. Review.
-
Unique, long-term effects of nicotine on adolescent brain.Pharmacol Biochem Behav. 2020 Oct;197:173010. doi: 10.1016/j.pbb.2020.173010. Epub 2020 Jul 30. Pharmacol Biochem Behav. 2020. PMID: 32738256 Free PMC article. Review.
-
Inhibition of 5α-reductase activity in late pregnancy decreases gestational length and fecundity and impairs object memory and central progestogen milieu of juvenile rat offspring.J Neuroendocrinol. 2011 Nov;23(11):1079-90. doi: 10.1111/j.1365-2826.2011.02219.x. J Neuroendocrinol. 2011. PMID: 21914008 Free PMC article.
-
Testing environment shape differentially modulates baseline and nicotine-induced changes in behavior: Sex differences, hypoactivity, and behavioral sensitization.Pharmacol Biochem Behav. 2018 Feb;165:14-24. doi: 10.1016/j.pbb.2017.12.003. Epub 2017 Dec 19. Pharmacol Biochem Behav. 2018. PMID: 29273458 Free PMC article.
-
Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug.Behav Brain Res. 2013 Nov 15;257:275-85. doi: 10.1016/j.bbr.2013.10.003. Epub 2013 Oct 10. Behav Brain Res. 2013. PMID: 24120402 Free PMC article.
References
-
- Adriani W, Macrì S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. - PubMed
-
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. - PubMed
-
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. - PubMed
-
- Balfour DJ, Khullar AK, Longden A. Effects of nicotine on plasma corticosterone and brain amines in stressed and unstressed rats. Pharmacol Biochem Behav. 1975;3:179–184. - PubMed
-
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

