Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme
- PMID: 20423964
- PMCID: PMC3191726
- DOI: 10.1136/bmj.c1804
Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme
Abstract
Objective: To assess the performance and impact of primary human papillomavirus (HPV) DNA screening with cytology triage compared with conventional cytology on cervical cancer and severe pre-cancerous lesions.
Design: Randomised trial.
Setting: Population based screening programme for cervical cancer in southern Finland in 2003-5.
Participants: 58 076 women, aged 30-60, invited to the routine population based screening programme for cervical cancer.
Interventions: Primary HPV DNA test (hybrid capture II) with cytology triage if the result was positive or conventional cytological screening (reference).
Main outcome measures: Rate of cervical cancer, cervical intraepithelial neoplasia (CIN) grade III, and adenocarcinoma in situ (as a composite outcome referred to as CIN III+) during 2003-7 through record linkage between files from the screening registry and the national cancer registry.
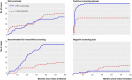
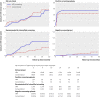
Results: In the HPV and conventional arms there were 95 600 and 95 700 woman years of follow-up and 76 and 53 cases of CIN III+, respectively (of which six and eight were cervical cancers). The relative rate of CIN III+ in the HPV arm versus the conventional arm was 1.44 (95% confidence interval 1.01 to 2.05) among all women invited for screening and 1.77 (1.16 to 2.74) among those who attended. Among women with a normal or negative test result, the relative rate of subsequent CIN III+ was 0.28 (0.04 to 1.17). The rate of cervical cancer between arms was 0.75 (0.25 to 2.16) among women invited for screening and 1.98 (0.52 to 9.38) among those who attended.
Conclusions: When incorporated into a well established organised screening programme, primary HPV screening with cytology triage was more sensitive than conventional cytology in detecting CIN III+ lesions. The number of cases of cervical cancer was small, but considering the high probability of progression of CIN III the findings are of importance regarding cancer prevention.
Trial registration: Current Controlled Trials ISRCTN23885553.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
References
-
- Screening for cancer of the uterine cervix. From the IARC working group on cervical cancer screening and the UICC project group on the evaluation of screening programmes for cancer. IARC Sci Publ 1986;76:1-315. - PubMed
-
- International Agency for Research on Cancer. IARC/WHO handbooks of cancer prevention. Vol 10: cervix cancer screening. IARC Press, 2005.
-
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. Vol 90: human papillomaviruses. IARC Press, 2007.
-
- Arbyn M, Dillner J, Schenck U, Nieminen P, Weiderpass E, Da Silva JD, et al. Methods for screening and diagnosis. In: Arbyn M, Anttila A, Jordan J, Ronco G, Segnan N, Wiener HG, eds. European guidelines for quality assurance in cervical cancer screening. 2nd ed. Office for Official Publications of the European Communities, 2008.