Structure-function studies of the SLC17 transporter sialin identify crucial residues and substrate-induced conformational changes
- PMID: 20424173
- PMCID: PMC2885210
- DOI: 10.1074/jbc.M110.130716
Structure-function studies of the SLC17 transporter sialin identify crucial residues and substrate-induced conformational changes
Abstract
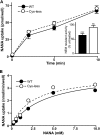
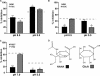
Salla disease and infantile sialic acid storage disorder are human diseases caused by loss of function of sialin, a lysosomal transporter that mediates H(+)-coupled symport of acidic sugars N-acetylneuraminic acid and glucuronic acid out of lysosomes. Along with the closely related vesicular glutamate transporters, sialin belongs to the SLC17 transporter family. Despite their critical role in health and disease, these proteins remain poorly understood both structurally and mechanistically. Here, we use substituted cysteine accessibility screening and radiotracer flux assays to evaluate experimentally a computationally generated three-dimensional structure model of sialin. According to this model, sialin consists of 12 transmembrane helices (TMs) with an overall architecture similar to that of the distantly related glycerol 3-phosphate transporter GlpT. We show that TM4 in sialin lines a large aqueous cavity that forms a part of the substrate permeation pathway and demonstrate substrate-induced alterations in accessibility of substituted cysteine residues in TM4. In addition, we demonstrate that one mutant, F179C, has a dramatically different effect on the apparent affinity and transport rate for N-acetylneuraminic acid and glucuronic acid, suggesting that it may be directly involved in substrate recognition and/or translocation. These findings offer a basis for further defining the transport mechanism of sialin and other SLC17 family members.
Figures




References
-
- Verheijen F. W., Verbeek E., Aula N., Beerens C. E., Havelaar A. C., Joosse M., Peltonen L., Aula P., Galjaard H., van der Spek P. J., Mancini G. M. (1999) Nat. Genet. 23, 462–465 - PubMed
-
- Wreden C. C., Wlizla M., Reimer R. J. (2005) J. Biol. Chem. 280, 1408–1416 - PubMed
-
- Reimer R. J., Edwards R. H. (2004) Pflugers Arch. 447, 629–635 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

