A small molecule inverse agonist for the human thyroid-stimulating hormone receptor
- PMID: 20427476
- PMCID: PMC2903937
- DOI: 10.1210/en.2010-0199
A small molecule inverse agonist for the human thyroid-stimulating hormone receptor
Abstract
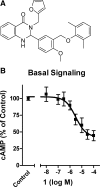
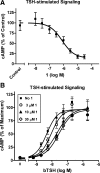
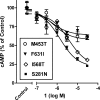
Small molecule inverse agonists for the TSH receptor (TSHR) may be used as probes of the role of basal (or agonist-independent or constitutive) signaling and may have therapeutic potential as orally active drugs to inhibit basal signaling in patients with thyroid cancer and in some patients with hyperthyroidism. We describe the first small-molecule ligand [1;2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one] that exhibits inverse agonist properties at TSHR. 1 inhibits basal and TSH-stimulated signaling, measured as cAMP production, by TSHRs in HEK-EM 293 cells stably expressing wild-type TSHRs; the antagonism of TSH-mediated signaling is competitive. 1 also inhibits basal signaling by wild-type TSHRs, and four constitutively active mutants of TSHR expressed transiently in HEK-EM 293 cells. 1 was active under more physiologically relevant conditions in primary cultures of human thyrocytes expressing endogenous TSHRs where it inhibited basal levels of mRNA transcripts for thyroglobulin, thyroperoxidase, sodium iodide symporter, and TSHR. These data serve as proof of principle that small, drug-like molecules can inhibit basal signaling by TSHR. We suggest that this small molecule is a lead compound for the development of higher-potency inverse agonists that can be used as probes of TSHR biology with therapeutic potential.
Figures
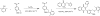

References
-
- Pierce KL, Premont RT, Lefkowitz RJ 2002 Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650 - PubMed
-
- Heitman LH, Ijzerman AP 2008 G protein-coupled receptors of the hypothalamic-pituitary-gonadal axis: a case for Gnrh, LH, FSH, and GPR54 receptor ligands. Med Res Rev 28:975–1011 - PubMed
-
- Vassart G, Dumont JE 1992 The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 - PubMed
-
- Kenakin T 2001 Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J 15:598–611 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

